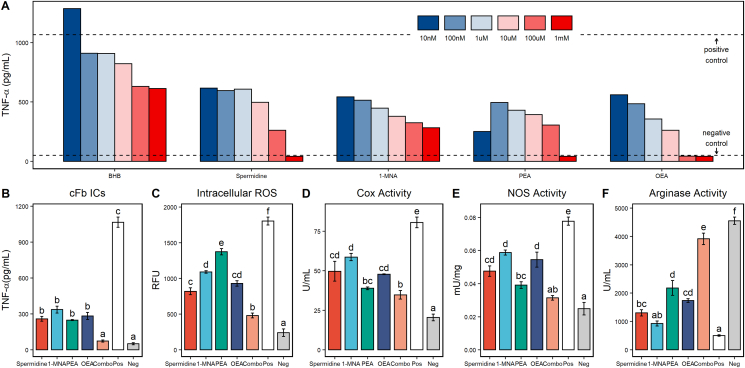
Figure 4.
Metabolites upregulated during fasting induce anti-inflammatory effects in macrophage. Tumor necrosis factor alpha (TNF-α) secretion from citrullinated fibrinogen immune complex (cFb IC)-stimulated primary human macrophage treated with beta-hydroxybutyrate (BHB), spermidine, 1-methylnicotinamide (1-MNA), palmitoylethanolamide (PEA), and oleoylethanolamide (OEA) at a final concentration of 1–10 nM (A). The TNF-α levels from unstimulated macrophage are shown as negative control values, and the TNF-α levels from stimulated macrophage without any other treatment are shown as positive control values. TNF-α secretion from cFb IC-stimulated primary human macrophage treated with spermidine (100 μM), 1-MNA (100 μM), PEA (10 μM), OEA (10 μM), and a combination treatment (combo) of all 4 metabolites at their individual concentrations (B). These concentrations were used for all vitro analyses (B–F). The negative and positive control values for all in vitro metabolite analyses (B–F) were generated from unstimulated macrophage or stimulated macrophage with no other treatment, respectively, as described earlier. Significance between treatments and controls for all in vitro analyses (B–F) is denoted by their assigned letter (a–f) where groups that share an assigned letter in common were found to have no significant differences (P > 0.05) and groups that do not share an assigned letter in common were found to be significantly different (P < 0.05) from each other. Significance was determined using Tukey’s test and is provided for Figure B–F for each treatment group versus the positive control. Positive control, 1065 ± 42.61 pg/mL; spermidine, 259 ± 19 pg/mL, P < 0.0001; 1-MNA, 336 ± 29 pg/mL, P < 0.0001; PEA, 248 ± 5 pg/mL, P < 0.0001; OEA, 283 ± 29 pg/mL, P < 0.0001; combo, 74 ± 9 pg/mL, P < 0.0001; and negative control, 52 ± 8 pg/mL. Intracellular ROS production from hydrogen peroxide-stimulated primary human macrophage treated with spermidine, 1-MNA, PEA, OEA, and combo (C). Positive control, 1804 ± 55 RFU; spermidine, 820 ± 47 RFU, P < 0.0001; 1-MNA, 1091 ± 18 RFU, P < 0.0001; PEA, 1375 ± 42 RFU, P = 0.0002; OEA, 930 ± 36 RFU, P < 0.0001; combo, 481 ± 32 RFU, P < 0.0001; and negative control, 241 ± 53 RFU. Total cyclooxygenase (COX) activity from lipopolysaccharide (LPS)-stimulated primary macrophage treated with spermidine, 1-MNA, PEA, OEA, and combo (D). Positive control, 80.6 ± 3.4 U/mL; spermidine, 49.7 ± 6.2 U/mL, P = 0.001; 1-MNA, 58.6 ± 2.3 U/mL, P = 0.0012; PEA, 38.9 ± 0.9 U/mL, P < 0.0001; OEA, 47.8 ± 0.2 U/mL, P < 0.0001; combo, 34.7 ± 2.7 U/mL, P < 0.0001; and negative control, 20.5 ± 2.1 U/mL. Total NOS activity from LPS- and interferon gamma (INF-γ)-stimulated THP-1 macrophage treated with spermidine, 1-MNA, PEA, OEA, and combo (E). Positive control, 0.076 ± 0.002 mU/mg; spermidine, 0.046 ± 0.003 mU/mg, P = 0.001; 1-MNA, 0.059 ± 0.002 mU/mg, P = 0.0034; PEA, 0.039 ± 0.002 mU/mg, P < 0.0001; OEA, 0.054 ± 0.004 mU/mg, P = 0.0009; combo, 0.031 ± 0.001 mU/mg, P < 0.0001; and negative control, 0.025 ± 0.004 mU/mg. Arginase activity from LPS- and INF-γ-stimulated THP-1 macrophage treated with spermidine, 1-MNA, PEA, OEA, and combo (F). Positive control, 506 ± 26 U/mL; spermidine, 1032 ± 110 U/mL, P = 0.01; 1-MNA, 927 ± 87 U/mL, P = 0.1836; PEA, 2181 ± 260 U/mL, P < 0.0001; OEA, 1736 ± 68 U/mL, P = 0.0007; combo, 3914 ± 203 U/mL, P < 0.0001; and negative control, 4550 ± 129 U/mL. All analyses described earlier were performed in duplicate using primary human macrophage isolated from a single healthy donor (A–D) or differentiated THP-1 macrophage (E–F).