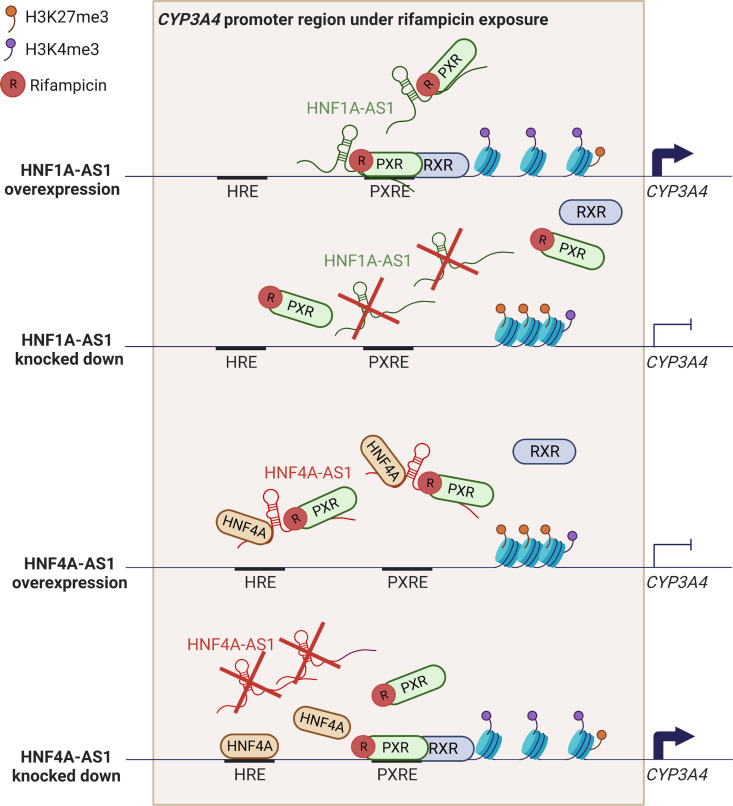
Fig. 4.
Possible mechanism of HNF1A-AS1 and HNF4A-AS1 in the regulation of rifampicin-induced expression of CYP3A4. HNF1A-AS1 can act as a scaffold to recruit activated PXR and guide it to the PXRE, where PXR forms a heterodimer with RXR to boost CYP3A4 expression. When overexpressing HNF1A-AS1, more activated PXR can be led to the PXRE so that H3K4me3 level increases and H3K27me3 decreases. CYP3A4 expresses more because of chromatin decondensation in its gene promoter region. On the contrary, knocking down HNF1A-AS1 can destroy the guidance for PXR to the PXRE, which leads to decreased H3K4me3 and increased H3K27me3 to compress the chromatin in the promoter of CYP3A4 and repress its expression. HNF4A-AS1 exhibits a different regulation mechanism of CYP3A4. It can function as a central binding platform for silencing HNF4A and PXR to inhibit CYP3A4 expression. When overexpressing HNF4A-AS1, more HNF4A and PXR are captured by HNF4A-AS1 and silenced, so they cannot bind to the HNF4A response element and the PXRE, which decreases H3K4me3 and increases H3K27me3 to inhibit CYP3A4. On the opposite, knocking down HNF4A-AS1 can destroy the central platform of HNF4A and PXR, which leads to more HNF4A and PXR binding to their binding sites and increase H3K4me3 and decrease H3K27me3 to decondense the chromatin in the promoter of CYP3A4 and promote its expression.