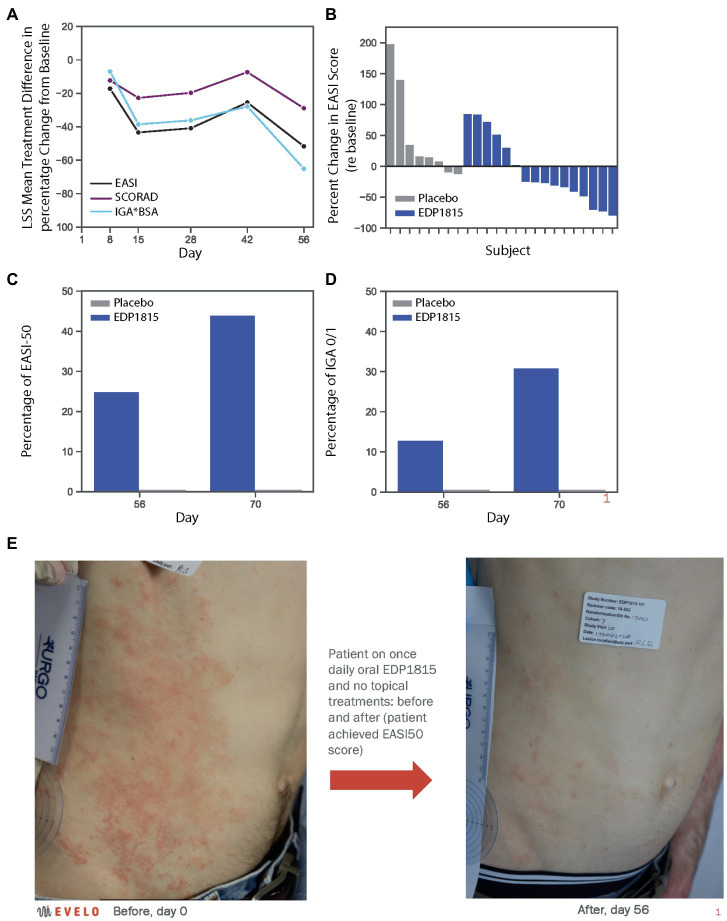
Figure 4.
EDP1815 leads to clinical improvements in a randomized double-blind trial of patients with atopic dermatitis. A phase 1b cohort of 24 patients with mild and moderate atopic dermatitis were randomized to EDP1815 (n = 16) or placebo (n = 8) and treated once daily for 56 days, with follow-up off treatment at day 70. (A) Clinical parameters of atopic dermatitis were measured at baseline and treatment days 8, 15, 28, 42, and 56. The treatment difference was calculated by subtracting the mean percentage change from baseline in placebo patients from that in active patients at each time point, and for each of the key clinical scores quantifying disease severity: EASI, IGA*BSA, and SCORAD. At day 56, the treatment difference for EASI was 52% (p = 0.062), for IGA*BSA was 65% (p = 0.022), and for SCORAD was 35% (p = 0.068). (B) Waterfall plot, with each participant’s percentage change from baseline in the EASI score at day 56 represented by each bar. Two placebo patients saw improvement, compared to ten patients randomized to EDP1815, with 4 patients achieving EASI50 or better at this timepoint. (C) Proportion of patients achieving EASI50 threshold or better, in active versus placebo group patients at day 56 (25% vs. 0%, respectively) and day 70 (44% vs. 0%, respectively). (D) Proportion of patients achieving IGA0/1 threshold in active versus placebo group patients at day 56 (13% vs. 0%, respectively) and day 70 (31% vs. 0%, respectively). (E) Photographs taken of a subject receiving EDP1815 and no topical or other active atopic dermatitis treatment in this study, at baseline and after 56 days of treatment. Significant improvements in erythema, papulation and excoriations are visible. The patient achieved an EASI improvement of 50%, from 9.8 at baseline to 4.9 at day 56.