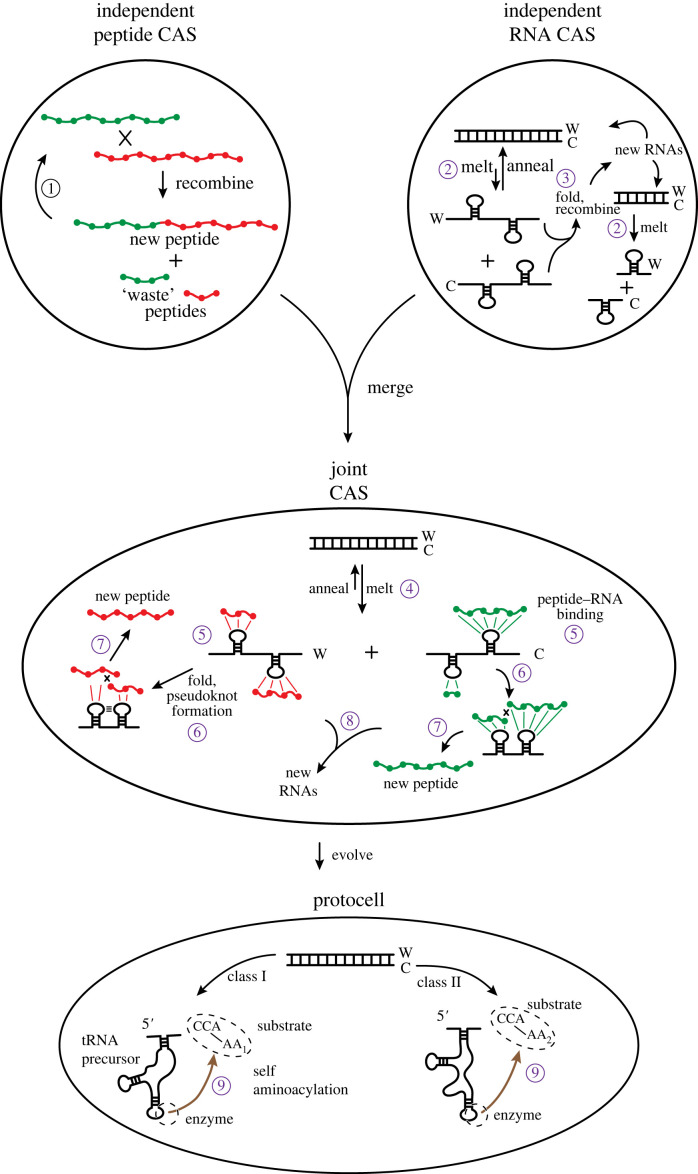
Figure 3.
Scenario for the origins of coding. Originally there are two independent CASs, a peptide CAS and an RNA CAS. In each, reproduction is possible on its own. In the peptide CAS, two peptides, green and red, if of sufficient length have the capacity to recombine to make new peptides. Small peptides of length, say, five amino acids or fewer do not possess sufficient complexity to recombine (or ligate, following [25]). They are ‘waste’ peptides, having low affinity for other short peptides. ① Longer products of reactions interact with other long products to create reaction cycles. Similar processes occur in the RNA CAS [24]. ② However, in the RNA CAS, there are two complementary strands of RNA upon annealing, a ‘Watson’ strand (W) and a ‘Crick’ strand (C). Annealing is favoured when two such strands interact; dsRNA also helps protect the RNA information from spontaneous hydrolysis. ③ If RNAs do melt, longer strands can adopt secondary structures including stem loops that engender catalytic activity. Shorter RNAs melt more easily, but have less tendency to possess catalytic activity. In both the independent CASs, reproduction is slow, sub-exponential, because of the structural, kinetic and thermodynamic barriers to proper catalytic events. Nevertheless, each CAS can operate without regard for the other. If, however, the two CASs find themselves in a situation in which they can interact productively, then the stage is set for coding, and life. In a joint CAS, each polymer provides assistance to the other, enhancing the other's reproductive rate. ④ Interaction with peptides shifts the equilibrium of the dsRNA to the melted, and potentially catalytically active, forms: ⑤ peptides, long or short, can bind to the loop regions of melted RNA strands. ⑥ The folding of single-stranded RNA into more complex stem loops and pseudoknots helps bring the peptides, including the shorter ones, into close contact so that ⑦ they can catalytically recombine/ligate to form larger peptides. One subset of peptides develops a tendency to bind the W strand of RNA, while another subset develops a tendency to bind the C strand. The advent of exactly two distinct subsets is the result of there being exactly two strands of RNA that form dsRNA [12]; dsRNA is the most thermodynamically stable nucleic acid complex. ⑧ Recombination/ligation of the RNAs proceeds as in the independent CAS case, although now its rate is augmented, eventually (through group selection) becoming exponential. Precursors to tRNAs could have arisen by the recombination of two similar stem loops [37,38]. Likewise, peptide reproduction is augmented because of the ability of smaller peptides to participate in the catalytic cycle. In the bottom, protocell stage, evolution drives the formation of the precursors to modern-day RNAs and proteins. Shorter and shorter peptides are selected for RNA loop binding, as the march to a 1 : 1 specificity between amino acids and nucleotide (triplets) is favoured by further reduction of waste (see text). ⑨ The RNAs are selected to covalently attach the single amino acids to their ends, forming the aminoacyl adenylates that are now palimpsests of this era. Coding arises in full when a unique associate between specific amino acids and specific RNA sequences becomes established; life is a consequence of the duality of polymer types and their association through this coding. Self-aminoacylation is the first aaRS function, shared with tRNA function in this model; the set of reproducing RNAs includes the precursors to tRNA, rRNA and aaRS activities, which later diverge and/or become taken over by peptides. The W and C strands of RNA each drive the evolution of the class I and II aaRS enzymes [12]. At each stage in this scenario, selection for the reproduction of the whole drives the evolutionary relationships of the parts (i.e. a Kantian whole).