ABSTRACT
Mediastinal masses can compress the respiratory or cardiovascular system, especially when anteriorly located. Obtaining histological material for diagnosis poses a challenge due to the major risk of cardiorespiratory collapse following anesthetic procedure. Our case shows the utility of rescue with venovenous extracorporeal membrane oxygenation (VV-ECMO) after occurrence of such an event and demonstrates the feasibility of administering chemotherapy during VV-ECMO. A 4-year-old boy was referred to the pediatric oncology clinic of our hospital after a large mediastinal mass was observed on chest radiography ordered due to persistent cough. Computed tomography of the thorax revealed a 100×85 mm mass in the anterior mediastinum, surrounding the heart, and showed that there was compression to the trachea, bronchiole, and vascular structures. Percutaneous needle biopsy accompanied by ultrasonography was planned for diagnostic purposes. Low-dose ketamine and midazolam were administered for procedural sedation in the operating room. After the biopsy procedure, the patient developed sudden airway obstruction requiring intubation. Despite 100% oxygen support with a mechanical ventilator, pulse oximeter saturation remained below 80%. Chest X-ray revealed total collapse of the left lung, and the patient’s oxygen saturation did not increase with selective left bronchial intubation. Bi-caval dual-lumen ECMO cannula was placed in the internal jugular vein and VV-ECMO was initiated, resulting in swift improvement in hypoxemia. The patients’s anterior mediastinal mass shrank rapidly and left lung improved with chemotherapy. The patient remained on ECMO for a total of 9 days and was extubated 2 days after ECMO termination, followed by discharge to the pediatric oncology ward on the 20th day of pediatric intensive care unit stay. It is well known that large, anteriorly-located mediastinal masses carry a considerable risk of causing cardio-pulmonary collapse during procedures involving anesthesia. All life-saving options, including emergency ECMO, should be available before any planned invasive procedures in these patients.
Keywords: Chemotherapy, extracorporeal membrane oxygenation, mediastinal mass, non-hodgkin lymphoma, pediatric intensive care
INTRODUCTION
Many pediatric malignancies may present with a mass in the mediastinal area. Certain tumors, especially those located in the anterior mediastinal area such as non-Hodgkin’s lymphoma (NHL), can compress the tracheobronchial system, as well as the heart and the large vessels associated with the heart.[1] It is known that general anesthesia during diagnostic core needle biopsy or definitive resection can lead to acute catastrophic airway collapse, which is very difficult to manage, especially in children.[2,3] After collapse of the airway, tracheal or selective bronchial intubation or emergent tracheostomy may not be successful. In such cases, application of extracorporeal membrane oxygenation (ECMO) until the compression effect caused by the tumor can be eliminated, may be life-saving.[4,5] There are numerous reports of ECMO utilization in cardiorespiratory collapse caused by mediastinal mass in adults.[6,7] However, even if it is suggested that pediatric cases with mediastinal mass have a greater risk of cardiopulmonary complications than adults, the experience pertaining to ECMO support in the management of such complications among children is limited.[8–11]
We report a case of a pediatric patient with a large anterior mediastinal mass (AMM), later diagnosed as NHL, that resulted in airway collapse during the diagnostic biopsy procedure which was performed with minimal anesthesia after initial attempts with local anesthesia failed. The airway could not be improved through usual approaches and hypoxemia ensued, ultimately resulting in the need for venovenous ECMO (VV-ECMO) support. Informed consent was obtained from the patient’s parents for publication.
CASE REPORT
A 4-year-old boy presented with a 10-day history of gradually increasing cough. He was initially diagnosed with pneumonia by his pediatrician and received sefuroxime treatment. Because his symptoms persisted without any improvement, a chest radiograph was obtained and revealed a large mediastinal mass (Fig. 1).
Figure 1.
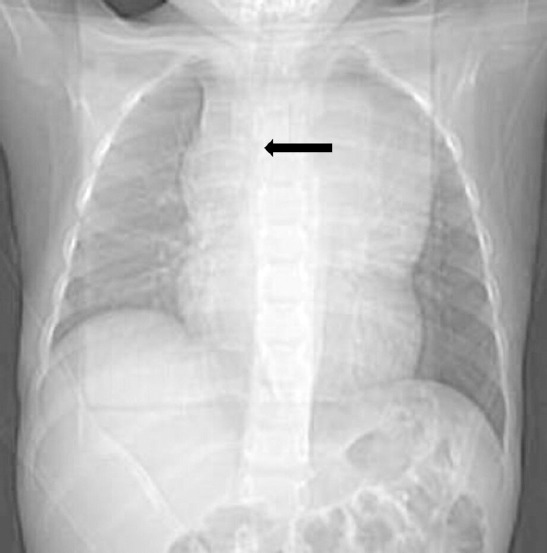
Initial chest radiography shows a wide mediastinum. The lower tracheal was pushed to the right side (black arrow).
The patient was referred to the pediatric oncology clinic of our hospital with a preliminary diagnosis of malignancy. Computed tomography (CT) of the thorax revealed a 100×85 mm mediastinal mass localized in the superior anterior area that was surrounding the heart and was compressing the right brachiocephalicus truncus, the left common carotid artery, the left subclavian artery, and arcus aorta. Predicted tracheal cross-section area (CSA) measurement was 40% (Fig. 2). Physical examination revealed clear respiratory sounds bilaterally, heart sounds were rhythmic, and there was no cardiac murmur. Venous distension was not present in the neck and there were no intercostal or subcostal retractions. Vital signs showed a body temperature of 36.6°C, pulse oxygen saturation of 96% in room air, respiration rate of 24/min, heart rate of 110/min, and a blood pressure of 92/65 mmHg. Initial laboratory results revealed a white blood cell count of 4.44×103/mm3 with 49.7% neutrophils, 34.6% lymphocytes, 9.3% monocytes, and 3% atypical lymphocytes, without any leukemic blasts. Venous blood gas examination showed pH: 7.38, PCO2: 39 mmHg, PO2: 41.2 mmHg, HCO3: 21 mmol/L, and a base deficit of –3.2 mmol/L.
Figure 2.
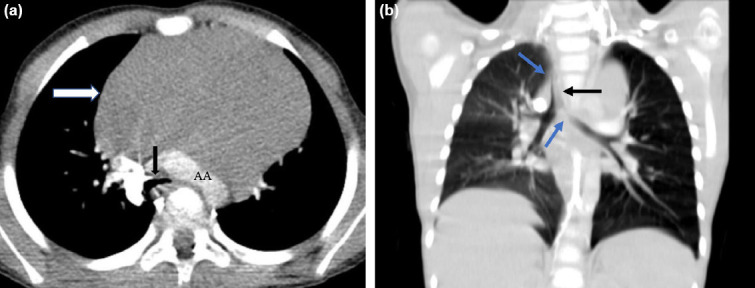
Initial chest computed tomography scan. On image (a), superior anterior mediastinal mass encases pulmonary arteries, trachea, arcus aorta, and bronchus in transverse view. The black arrow indicates the trachea at this point of maximal compression. The predictive cross-sectional area mesurement is estimated to be 40%. The white arrow points to the mediastinal mass, which is homogeneous nature. On image (b), mediastinal mass compresses left mainstream bronchus and trachea (blue arrows) and pushes the trachea to the right (black arrow) in coronal view.
Diagnostic percutaneous core needle biopsy was planned. Although respiratory symptoms of the patient were minimal, the patient was defined to have high risk for the anesthesia procedure due to a predicted CSA measurement of <50% and accompanying left main bronchus compression. The pediatric intensive care unit (PICU) team was informed about the patient, and the initial decision was to try and undertake the biopsy procedure under local anesthesia. However, the child was uncooperative and all attempts were unsuccessful. With a decision to utilize minimal anesthesia, one dose ketamine and midazolam were given as procedural sedoanalgesia. Accompanied by ultrasonography, tru-cut biopsy was performed by entering through the left lateral thorax wall in the left lateral position. Shortly after the procedure, pulse oxygen saturation dropped below 80% and oxygen saturation did not increase despite bag-mask ventilation and oxygen support; thus, the patient was intubated and transferred to the PICU without delay.
In the PICU, mechanical ventilator support and 100% oxygen still could not improve pulse oxygen saturation above 75%, and the arterial blood gas analysis of the patient revealed pH: 7.37, PCO2: 42 mmHg, PO2: 44 mmHg, HCO3: 18 mmol/L, and base deficit was -6.4 mmol/L. Chest radiography revealed total collapse in the right lung and hyperaeration in the left lung (Fig. 3). Echocardiography demonstrated normal ventricular function without major external compression in the great arteries. Selective left bronchial intubation was planned using a double-lumen endotracheal tube to ventilate the collapsed lung. However, severe external compression of the left main bronchus, resulting in slit-like opening, distal collapse, and acute angulation away from the carina, prevented the advancement of the endotracheal tube past the site of compression. The patient was placed in the left lateral recumbent position to reduce mass-related compression on the lung, but there was no change in pulse oxygen saturation. Saturation values ranged between 68% and 73% despite 100% oxygen support, indicating a decreasing trend. On detection of pH: 7.26, PCO2: 61 mmHg, PO2: 45 mmHg, HCO3: 18 mmol/L, and a base deficit of –7.2 mmol/L in arterial blood gas, we decided to apply VV-ECMO support at the 3rd h of admission to the PICU. A 19 Fr. Avalon Elite Bi-Caval Dual-Lumen ECMO cannula was placed by the pediatric intensivist into the right internal jugular vein of the patient through the Seldinger technique under the guidance of transthoracic echocardiography. The placement and position of the ECMO cannula was confirmed by chest radiography (Fig. 4). The ECMO circuit consisted of a 1.8 m2 Maquet polymethylpentene membrane oxygenator with ¼ inch connector lines, a maquet rota-flow centrifugal pump, and a maquet permanent life support ECMO set (Maquet Cardiovascular, Wayne, NJ, USA). The patient remained hemodynamically stable for the duration of the procedure. After completion of the procedure, ECMO was initiated with 500 revolutions per minute (RPM) and increased by incremental steps of 500 RPM until blood flow rate reached 90 mL/kg/min.
Figure 3.
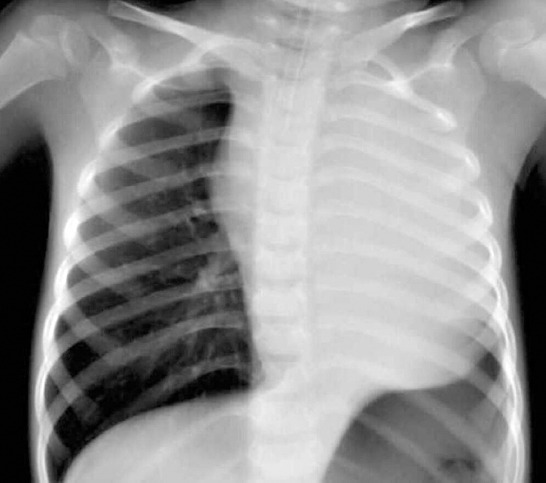
Chest radiography shows total left lung collapse.
Figure 4.
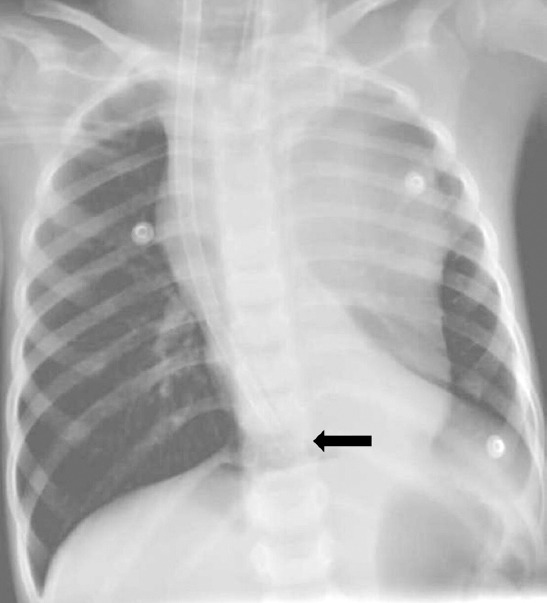
Avalon Elite® is a bicaval dual lumen venovenous ECMO cannula inserted through the right internal jugular vein. The distal end of the cannula is located in the cavoatrial junction (black arrow).
After ECMO application, the pulse oxygen saturation of the patient exceeded 95% with 90% oxygen support. Signs of hypoxemia regressed in blood gas analysis. Lung-protective mechanical ventilation strategy was applied with target saturation values above 82%. To ensure safe ECMO operation, activated clotting time values were maintained between 180 and 220 seconds, and no significant bleeding complications were recorded.
Pathological examination of the frozen biopsy tissue was consistent with NHL, ultimately defined as a T-cell lymphoblastic lymphoma. While on VV-ECMO, the patient received intravenous chemotherapy for NHL (CHOP protocol) consisting of high-dose steroids, cyclophosphamide, daunorubicin, and vincristine.[1] He received standard hyperhydration and alkalization. Lung radiography showed rapid shrinkage of the mediastinal mass in response to chemotherapy induction. The ECMO weaning process was initiated on the 5th day of ECMO. On day 9 of ECMO support, the patient was completely weaned off ECMO and decanulated (Fig. 5). He was extubated 2 days later and discharged to the pediatric oncology ward on the 20th day of PICU stay.
Figure 5.
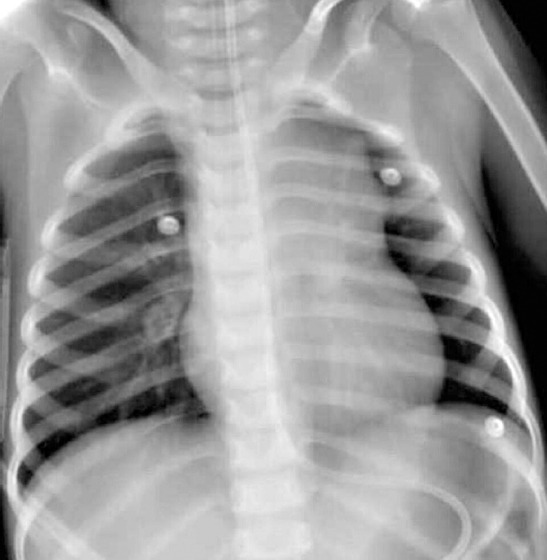
Chest radiography after ECMO decannulation. ECMO: Extracorporeal membraneoxygenation.
DISCUSSION
Here, we described an effective and life-saving application of VV-ECMO to a pediatric patient with an AMM who developed sudden airway collapse after the use of sedoanalgesic medication for core needle biopsy.
Presentation characteristics of mediastinal mass may vary greatly in all ages, depending on size, location and underlying pathology, and some can be asymptomatic. Around half of adult patients with mediastinal mass are reported to be asymptomatic or present with dyspnea and/or chest pain.[2] In the pediatric age group, patients may be admitted with non-specific symptoms, such as cough, similar to our patient, and mediastinal mass may be incidentally identified through X-ray ordered for other causes.[2,3] Infants and small children may be more susceptible than adults to external airway obstruction, because their cartilaginous airways are relatively more compressible. Furthermore, small decreases in airway diameter create a relatively larger decrease in overall luminal area and can increase airway resistance to a greater degree.[12]
Mediastinal masses, especially those located anteriorly, are known to have respiratory or cardiovascular risks during anesthesia or diagnostic and/or curative procedures.[12–14] Patients are typically in the supine position during procedures, thereby increasing the possibility of greater compression from the weight of the mass. With induction of anesthesia, the tone of the respiratory muscles, including the diaphragm and intercostal muscles, are decreased – which compromises functional residual capacity. Subsequently, the transmural pressure decreases and the pleural pressure becomes less negative, thereby increasing the risk for further extrinsic compression during anesthesia.[5,15] Minimal anesthesia intervention (preferably local anesthesia) and maintenance of spontaneous respiration are suggested in high-risk patients. However, this requires a significant degree of cooperation from the patient, and the chance of converting to general anesthesia is reportedly very high.[14]
There is a moderate amount of the literature on this topic, but these studies have a number of limitations. The relative rarity of the problem results in there being few pediatric series, even though children may actually be at greater risk than adults for anesthesia-related respiratory complications in such cases.[8,14] There is a risk for underestimation of the severity of the problem since pulmonary reserves may not be determined accurately, patient history is often insufficient, and there are very few studies emphasizing the importance of the weaker structure of pediatric patients’ elastic cartilaginous airways. Due to these risks, researchers have sought to define accurate ways to measure preoperative cardiovascular risk in patients with mediastinal masses using clinical findings together with several diagnostic investigations, such as pulmonary function tests (PFTs), tracheal and bronchial CSA measurements, and mass/chest diameter ratio.[4,16,17] In addition, the presence of orthopnea and stridor has been identified as key features that were significantly associated with the risk for anesthetic complication in pediatric patients with AMM.[18,19] However, due to the non-specific nature of the symptoms and the limited ability of the patient to identify symptoms particularly in the younger age group, performing cardiopulmonary risk analysis with only clinical findings may not be sufficient. We also believe it to be important to note that, although our patient did not have severe respiratory symptoms such as dyspnea, orthopnea or stridor, sudden airway collapse still developed during the procedure, indicating that the absence of such features does not guarantee safety.
PFTs, which are used for the assessment of anesthesia-related risks in adults, may not be feasible in children due to the lack of patient cooperation. Thorax CT, on the other hand, may be a more objective modality for risk assessment in both adults and children. Mass/chest ratio, expressed as the diameter of the mass to the diameter of the chest, is one of the measurements that can be obtained from thorax CT. Anesthesia-related complication risk is defined to be low if the ratio is less than 33%; whereas, if the ratio is greater than 45%, the patient is defined to be at high risk. Another measure that can be obtained from CT is the two-dimensional measurement of trachea CSA. The percentage of predicted CSA is obtained by comparing the CSA measured at the narrowest site to the CSA measured in a non-compressed area. It is stated that predicted CSA values of <50% (in other words, presence of a tracheal compression ratio of ≥50%) increase the risk of life-threatening respiratory complications.[12,14,15] Our case was initially considered to be have high risk for anesthesia-related complications, because predicted CSA measurement was approximately 40% in thorax CT evaluation and this was accompanied by left main bronchus compression.
Based on the critical nature of mediastinal masses, Hack et al.[18] proposed pre-operative anesthetic guidelines for high-risk patients; they emphasized that physicians should aim to maintain spontaneous breathing as much as possible, while also avoiding the use of long-acting relaxants to allow a rapid return to spontaneous breathing. It was also noted that changing patient position to alleviate anterior compression would be beneficial. Therefore, it is evident that utilizing local or regional anesthetic techniques is critical to minimize the need for systemic anesthesia in high-risk patients with mediastinal mass.[14,20] Tanaka et al.[14] reported safe diagnostic management experiences in pediatric patients with mediastinal tumors with signs of respiratory distress. In their study, diagnostic needle biopsy was applied with local anesthesia, and no complications were reported in five out of the 12 patients. The resection procedure was performed under general anesthesia in one patient and the remaining patients were diagnosed through lower-risk diagnostic procedures, such as peripheral blood smears, flow cytometry, and tumor markers. Another study, by Malik et al.[21] reported the application of a combinatory approach to prevent complications, including utilization of local anesthetic strategies, placement of patients in the lateral or sitting position during intervention, maintenance of spontaneous breathing, and use of ketamine and minimizing muscle relaxants, in their study of 44 pediatric patients with AMM. They reported no major anesthesia-related complications. However, even if any and all precautions are taken to prevent complications, such events may still occur, as was the case in our patient. In the literature, the risk of anesthesia-related complications in children with AMM has been reported to vary between 5 and 18%, and oxygen desaturation has been identified as the most common complication.[16,18,19,22]
A needle biopsy was planned under local anesthesia since our patient was in the high-risk group for anesthetic procedure; but attempts were unsuccessful due to inadequate patient cooperation. As a secondary option, low-dose ketamine and midazolam were applied as per the suggestions of the Procedural Sedation (Conscious) and Analgesia Guideline, in-line with the purpose of obtaining biopsy without suppressing spontaneous breathing.[23] Despite applying minimal sedation and ensuring the continuation of spontaneous breathing, our patient developed life-threatening sudden airway collapse.
There are numerous cases of successful ECMO application in adult patients with acute cardiopulmonary collapse due to AMM-caused compression of extrinsic airways and/or main vessels.[8,24–26] In the neonatal period, ECMO applications due to mediastinal mass are much less common, and the etiology of applications includes congenital malformations (such as congenital cystic adenomatoid malformations) and, much more rarely, congenital tumors.[17,27] The experience related to the management of mass-caused acute cardiopulmonary collapse with ECMO application is limited since there are only a small number of cases in the pediatric age group. In fact, a literature review revealed five published cases of pediatric patients, in which ECMO was utilized in the management of complications caused by mediastinal mass. These cases are summarized in Table 1.[8–11]
Table 1.
Literature review of pediatric patients who underwent ECMO due to mediastinal masses
| Author(s) / year of publication | Age (years) | Sex | Underlying diagnoses | The origin of the mass | Reason for ECMO | ECMO type | Duration of ECMO (day) | Survival |
|---|---|---|---|---|---|---|---|---|
| 1, Frey 9, 2006 | 10 | Male | T-cell non-hodgkin lymphoma | Anterior mediastinal mass | Cardiovascular collapse | VA- ECMO | 6 | Alive |
| 2, Au 10, 2020 | 4 | Male | Esophageal duplication cyst | Posterior mediastinal mass | Airway collapse | VV-ECMO | 2 | Alive |
| 3, Huang 8, 2010 | 15 | Female | T-cell acute lypmhoblastic leukemia | Anterior mediastinal mass | Cardiovascular collapse | VA-ECMO | 2 | Dead |
| 4, Wickiser 11, 2006 | 11 | Male | T-cell acute lypmhoblastic leukemia | Anterior mediastinal mass | Airway collapse | VA- ECMO | 6 | Alive |
| 5, Wickiser 11, 2006 | 4 | Male | T-cell non-hodgkin lymphoma | Anterior mediastinal mass | Airway collapse | VA-ECMO | 4 | Alive |
| 6, Present case | 4 | Male | T-cell non-hodgkin lymphoma | Anterior mediastinal mass | Airway collapse | VV-ECMO | 9 | Alive |
ECMO: Extracorporeal membrane oxygenation; VA: Veno-arterial; VV: Veno-venous.
Despite the fact that mediastinal masses are recognized as being an indication for ECMO, there are no reports on this issue in the database of the extracorporeal life support organization.[28] In our patient, emergency VV-ECMO was applied as a life-saving bridge treatment until tumor mass could be reduced via chemotherapy or debulking surgery. The complication leading to the use of ECMO was sudden airway collapse during minimal sedation for a diagnostic procedure, and the need for ECMO became apparent as a result of failure to obtain respiratory improvement with conventional interventions and maneuvers. Chemotherapy induction was preferred instead of debulking surgery to reduce the size of the mass since NHL is typically sensitive to chemotherapy. In-line with expectations, chemotherapy resulted in a swift and significant reduction of mass volume, subsequently leading to the alleviation of compression and the regression of lung collapse. The patient was successfully separated from ECMO after 9 days with treatment, of which the past 4 days included weaning.
This case shows that, even when clinicians are well-aware of the risks and are careful in their preparation, patients with AMM may still suffer from catastrophic anesthesia-related cardiopulmonary complications. In other words, utilizing minimal anesthesia and preserving spontaneous breathing during intervention procedures may not be sufficient to eliminate the risk of sudden airway collapse in cases with high risk for anesthesia-related complications. It should be kept in mind that endotracheal intubation, selective bronchial intubation or other approaches, and maneuvers may fail in such cases, and clinicians should be prepared for the worst-case scenario. We have demonstrated with this case that ECMO is a viable option that will allow maintenance of cardiopulmonary stability and respiratory support while the actual causative pathology – in this case, NHL – can be treated with chemotherapy or debulking. We utilized VV-ECMO over VA-ECMO due to absence of cardiac compromise and to avoid the risks of arterial cannulation.
Conclusion
Children with a large AMM remain as complicated cases that usually cause diagnostic and/or treatment-related challenges. Cardiopulmonary collapse can occur even when the patient receives minimal anesthesia. Compared with adults, pediatric patients appear to be at higher risk for intraoperative respiratory and cardiovascular complications and pose additional challenges to anesthesiologists due to anatomic, physiologic, and age-specific characteristics. We believe that this case underlines the recognized risks for patients with AMMs and demonstrates the utility and feasibility of rescue ECMO as a life-saving procedure that provides the necessary time for respiratory treatment. This report highlights the importance of considering ECMO (when available) for select cases that could be at risk for cardiopulmonary failure secondary to AMM.
Footnotes
Informed Consent: Written informed consent was obtained from the patient parents for the publication of the case report and the accompanying images.
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: M.D.; Design: M.D., Z.K.; Supervision: M.D.; Materials: M.D.; Data: M.D., Z.K.; Analysis: M.D., Z.K.; Literature search: M.D., Z.K.; Writing: M.D., Z.K.; Critical revision: M.D.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Linschoten M, Kamphuis JA, Van Rhenen A, Bosman LP, Cramer MJ, Doevendans PA, et al. Cardiovascular adverse events in patients with non-hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP):A systematic review and meta-analysis. Lancet Haematol. 2020;7:e295–308. doi: 10.1016/S2352-3026(20)30031-4. [DOI] [PubMed] [Google Scholar]
- 2.Azarow KS, Pearl RH, Zurcher R, Edwards FH, Cohen AJ. Primary mediastinal masses:A comparison of adult and pediatric populations. J Thorac Cardiovasc Surg. 1993;106:67–72. [PubMed] [Google Scholar]
- 3.Lam JCM, Chui CH, Jacobsen AS, Tan AM, Joseph VT. When is a mediastinal mass critical in a child?An analysis of 29 patients. Pediatr Surg Int. 2004;20:180–4. doi: 10.1007/s00383-004-1142-6. [DOI] [PubMed] [Google Scholar]
- 4.Blank RS, De Souza DG. Anesthetic management of patients with an anterior mediastinal mass:Continuing professional development. Can J Anaesth. 2011;58:853–9. doi: 10.1007/s12630-011-9539-x. [DOI] [PubMed] [Google Scholar]
- 5.Luckhaupt-Koch K. Mediastinal mass syndrome. Paediatr Anaesth. 2005;15:437–8. doi: 10.1111/j.1460-9592.2005.01614.x. [DOI] [PubMed] [Google Scholar]
- 6.Raza HA, Nokes BT, Jaroszewski D, Garrett A, Sista R, Ross J, et al. VV-ECMO for surgical cure of a critical central airway obstruction. Respir Med Case Rep. 2019;28:100890. doi: 10.1016/j.rmcr.2019.100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YL, Yang MC, Huang CH, Wang CC, Wu ET, Huang SC, et al. Rescue of cardiopulmonary collapse in anterior mediastinal tumor:Case presentation and review of literature. Pediatr Emerg Care. 2010;26:296–8. doi: 10.1097/PEC.0b013e3181d6daf0. [DOI] [PubMed] [Google Scholar]
- 8.Lueck C, Kuehn C, Hoeper MM, Ganser A, Eder M, Beutel G. Successful use of extracorporeal membrane oxygenation during induction chemotherapy in a patient with mediastinal tumor mass of a T lymphoblastic lymphoma. Ann Hematol. 2016;95:1719–21. doi: 10.1007/s00277-016-2734-7. [DOI] [PubMed] [Google Scholar]
- 9.Frey TK, Chopra A, Lin RJ, Levy RJ, Gruber P, Rheingold SR, et al. A child with anterior mediastinal mass supported with veno-arterial extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2006;7:479–81. doi: 10.1097/01.PCC.0000235247.10880.F8. [DOI] [PubMed] [Google Scholar]
- 10.Au V, Marsh B, Benkwitz C. Resection of a posterior mediastinal mass in a 4-year-old child complicated by difficult airway management and emergent use of extracorporeal membrane oxygenation. Semin Cardiothorac Vasc Anesth. 2020;24:349–54. doi: 10.1177/1089253220960267. [DOI] [PubMed] [Google Scholar]
- 11.Wickiser JE, Thompson M, Leavey PJ, Quinn CT, Garcia NM, Aquino VM. Extracorporeal membrane oxygenation (ECMO) initiation without intubation in two children with mediastinal malignancy. Pediatr Blood Cancer. 2007;49:751–4. doi: 10.1002/pbc.20741. [DOI] [PubMed] [Google Scholar]
- 12.Béchard P, Létourneau L, Lacasse Y, CôtéD , Bussières JS. Perioperative cardiorespiratory complications in adults with mediastinal mass. Anesthesiology. 2004;100:826–34. doi: 10.1097/00000542-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Hammer GB. Anaesthetic management for the child with a mediastinal mass. Paediatr Anaesth. 2004;14:95–7. doi: 10.1046/j.1460-9592.2003.01196.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Amano H, Tanaka Y, Takahashi Y, Tajiri T, Tainaka T, et al. Safe diagnostic management of malignant mediastinal tumors in the presence of respiratory distress:A 10-year experience. BMC Pediatr. 2020;20:292. doi: 10.1186/s12887-020-02183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anghelescu DL, Burgoyne LL, Liu T, Li CS, Pui CH, Hudson MM, et al. Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Paediatr Anaesth. 2007;17:1090–8. doi: 10.1111/j.1460-9592.2007.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garey CL, Laituri CA, Valusek PA, St Peter SD, Snyder CL. Management of anterior mediastinal masses in children. Eur J Pediatr Surg. 2011;21:310–3. doi: 10.1055/s-0031-1279745. [DOI] [PubMed] [Google Scholar]
- 17.Etches PC, Tiemey AJ, Demianczuk NN. Successful outcome in a case of cystic adenomatoid malformation of the lung complicated by fetal hydrops, using extracorporeal membrane oxygenation. Fetal Diagn Ther. 1994;9:88–91. doi: 10.1159/000263914. [DOI] [PubMed] [Google Scholar]
- 18.Hack HA, Wright NB, Wynn RF. The anaesthetic management of children with anterior mediastinal masses. Anaesthesia. 2008;63:837–46. doi: 10.1111/j.1365-2044.2008.05515.x. [DOI] [PubMed] [Google Scholar]
- 19.King DR, Patrick LE, Ginn-Pease ME, McCoy KS, Klopfenstein K, et al. Pulmonary function is compromised in children with mediastinal lymphoma. J Pediatr Surg. 1997;32:294–9. doi: 10.1016/s0022-3468(97)90197-4. ;discussion 299–300. [DOI] [PubMed] [Google Scholar]
- 20.Turoff RD, Gomez GA, Berjian R, Park JJ, Priore RL, Lawrence DD, et al. Postoperative respiratory complications in patients with Hodgkin's disease:Relationship to the size of the mediastinal tumor. Eur J Cancer Clin Oncol. 1985;21:1043–6. doi: 10.1016/0277-5379(85)90288-3. [DOI] [PubMed] [Google Scholar]
- 21.Malik R, Mullassery D, Kleine-Brueggeney M, Atra A, Gour A, Sunderland R, et al. Anterior mediastinal masses-A multidisciplinary pathway for safe diagnostic procedures. J Pediatr Surg. 2019;54:251–4. doi: 10.1016/j.jpedsurg.2018.10.080. [DOI] [PubMed] [Google Scholar]
- 22.Ng A, Bennett J, Bromley P, Davies P, Morland B. Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer. 2007;48:160–4. doi: 10.1002/pbc.20702. [DOI] [PubMed] [Google Scholar]
- 23.Practice guidelines for moderate procedural sedation and analgesia 2018:A report by the American society of anesthesiologists task force on moderate procedural sedation and analgesia the American association of oral and maxillofacial surgeons. American college of radiology American dental association American society of dentist anesthesiologists and society of interventional radiology Anesthesiology. 2018;128:437–79. doi: 10.1097/ALN.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AS, Smythe WR, Aukburg S, Kaiser LR, Fox KR, Bavaria JE. Severe acute extrinsic airway compression by mediastinal tumor successfully managed with extracorporeal membrane oxygenation. ASAIO J. 1998;44:219–21. doi: 10.1097/00002480-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Wohlfarth P, Ullrich R, Staudinger T, Bojic A, Robak O, Hermann A, et al. Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit Care. 2014;18:R20. doi: 10.1186/cc13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JJ, Moon SW, Kim YH, Choi SY, Jeong SC. Flexible bronchoscopic excision of a tracheal mass under extracorporeal membrane oxygenation. J Thorac Dis. 2015;7:E54–7. doi: 10.3978/j.issn.2072-1439.2015.01.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo T, Ando H, Watanabe Y, Harada T, Ito F, Kaneko K, et al. Acute respiratory failure associated with intrathoracic masses in neonates. J Pediatr Surg. 1999;34:1633–7. doi: 10.1016/s0022-3468(99)90632-2. [DOI] [PubMed] [Google Scholar]
- 28.Maratta C, Potera RM, Van Leeuwen G, Moya AC, Raman L, Annich GM. Extracorporeal life support organization (ELSO):2020 pediatric respiratory ELSO guideline. ASAIO J. 2020;66:975–9. doi: 10.1097/MAT.0000000000001223. [DOI] [PubMed] [Google Scholar]


