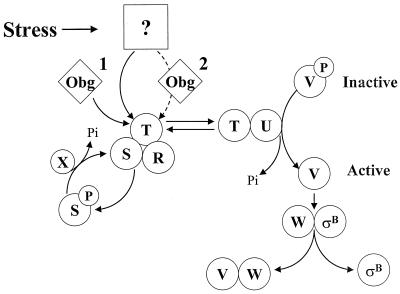
FIG. 1.
Model for stress activation of ςB. ςB is held inactive in unstressed B. subtilis as a complex with an anti-ςB protein, RsbW (W). ςB is freed from RsbW when a release factor, RsbV (V), binds to RsbW. In unstressed B. subtilis, RsbV is inactive due to an RsbW-catalyzed phosphorylation (V-P). Environmental stress activates an RsbV-P phosphatase, RsbU (U), which reactivates RsbV. RsbT (T) is the RsbU activator. RsbT is normally bound to a negative regulator, RsbS (S), which inhibits its activity. RsbR (R) also binds to RsbS and -T and is believed to facilitate their interactions. Upon exposure to stress, RsbT phosphorylates and inactivates RsbS and then activates the RsbU phosphatase. Obg, an essential GTP binding protein, is required for stress to trigger the activation of RsbT. It is unknown whether an Obg-dependent process is a cofactor for stress to activate RsbT (step 1) or whether stress communicates directly to RsbT through Obg (step 2). RsbS-P is dephosphorylated and reactivated by a phosphatase, RsbX (X), that is encoded by one of the genes downstream of the sigB operon's ςB-dependent promoter. RsbX levels become elevated when ςB is active, which may facilitate a return of RsbT to an inactive complex with RsbS. The model is based on references 1, 3, 5, 6, 8, 15, 27, 35, 38, and 42).