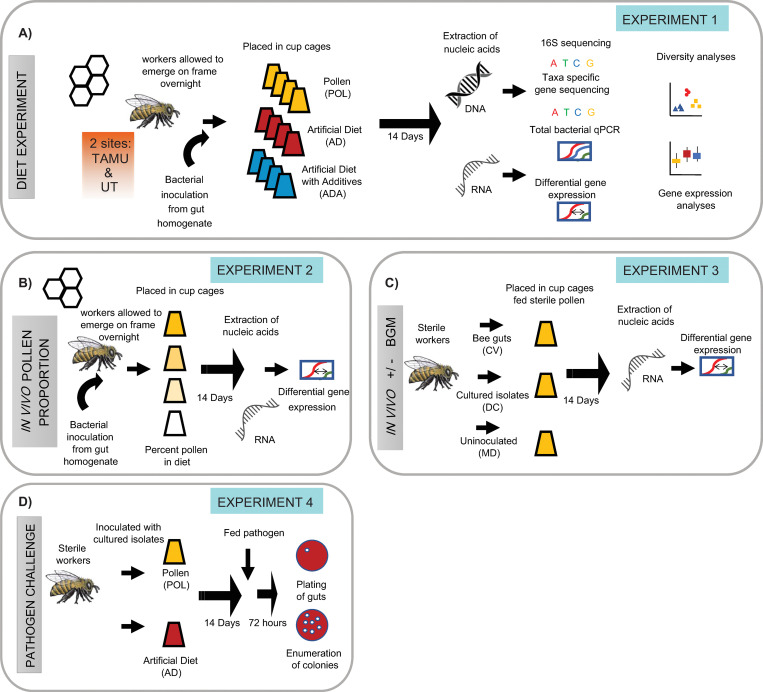
Fig 1. Design of experiments on effects of microbiota and diet.
(A) Effect of diet on the bee gut microbiota and gene expression. We allowed worker bees to emerge from a frame overnight and inoculated them with conventional honey bee gut microbiota. We placed them into cup cages and fed them either pollen (POL), an artificial diet (AD), or an artificial diet that included hemicellulose and pectin additives (ADA). After 14 days we dissected the bees’ abdomens and extracted DNA and RNA from 7 workers sampled from 4 cups per condition per site. We used these nucleic acids to perform 16S rRNA gene or taxon-specific gene sequencing, as well as quantitative polymerase chain reaction (qPCR) of total copies of 16S rRNA genes. We used RNA to make cDNA and used qPCR to examine transcript abundance of developmental and dietary genes. (B) Effect of proportion pollen in diet on expression of developmental genes. We allowed bees to emerge on a brood frame overnight and then conventionalized them by feeding them a gut homogenate prepared from adult workers. We placed the bees into cup cages and fed each cup a different proportion of pollen mixed with an artificial diet (percentage of pollen in cups = 100%, 50%, 25%, or 0%). After 14 days we dissected the bees’ abdomens and performed RNA extraction and developmental gene assays as above (n = 8 bees per condition). (C) Effect of microbiota on expression of developmental genes. We pulled late-stage pupae from brood frames and allowed them to emerge as adults in sterile conditions. These sterile workers were either inoculated with gut homogenate from adult bees (conventionalized = CV), with co-cultured gut bacterial isolates (defined community = DC), or kept uninoculated (microbiota deficient = MD). We placed the bees into cup cages with sterile pollen and sugar syrup for 14 days. We then dissected abdomens, extracted RNA, and used this to synthesize cDNA and run qPCR to examine developmental gene expression. This experiment was performed twice because the CV group died in the first round (n = 8 bees from 2 cups per condition, repeated twice). (D) Effect of diet on susceptibility to a bacterial pathogen. We generated sterile workers as in (C), placed 20 workers into 2 cups, and inoculated them with a co-cultured defined community of gut bacteria. We fed pollen to one of the cups and an artificial diet to the other one. After 14 days we fed each of the bees 5 μL from and OD600 = 1 suspension of Serratia marcescens (strain KZ11) tagged with a kanamycin-resistant marker. We maintained these bees for 72 h, dissected their guts, and then plated dilutions of the homogenate on kan+ plates. We counted the number of resultant colonies (n = 15 bees per condition).