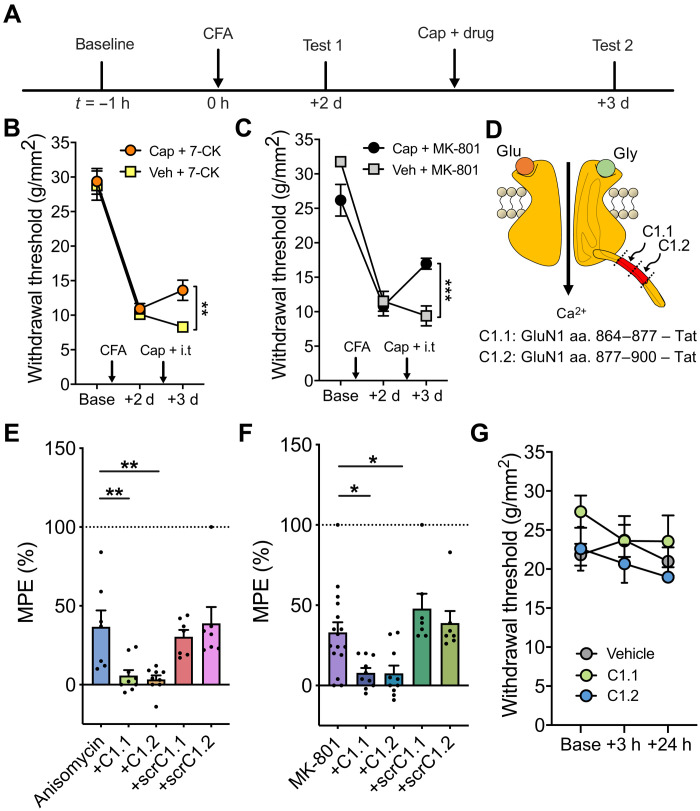
Fig. 2. NI-NMDAR activity and reactive destabilization produce long-lasting analgesic effects that are both dependent on GluN1 C-terminal interactions.
(A) Timeline of experimental protocol. (B and C) Changes in mechanical withdrawal thresholds induced by intraplantar CFA followed by a second ipsilateral intraplantar injection of capsaicin or vehicle and intrathecal injection of (B) 7-CK (NMDAR glycine site antagonist) or (C) MK-801 (NMDAR pore blocker). (D) Schematic diagram of GluN1 C-terminal target sites for C1.1 and C1.2 mimetic peptides. aa., amino acid. (E) Summary of antihyperalgesia induced by intrathecal injection of anisomycin alone, anisomycin + mimetic Tat peptides (C1.1 or C1.2), or anisomycin + scrambled peptide controls (scrC1.1 or scrC1.2), expressed as percentage of MPE. (F) Summary of antihyperalgesia induced by intrathecal injection of MK-801 alone, MK-801 + mimetic Tat peptides (C1.1 or C1.2), or MK-801 + scrambled peptide controls (scrC1.1 or scrC1.2), expressed as percentage of MPE. (G) Intrathecal injection of vehicle, C1.1, or C1.2 mimetic peptides had no effect on the withdrawal threshold of naïve C57BL/6 N mice. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.