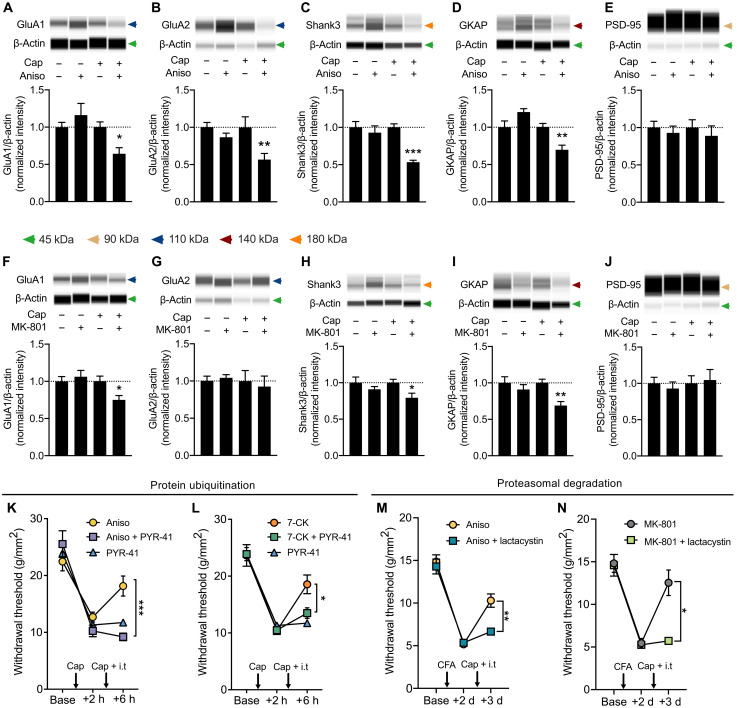
Fig. 4. Ubiquitin-dependent proteosomal degradation of postsynaptic proteins underlies the depotentiation of sensitized pain pathways following destabilization.
(A to E) CFA-induced hyperalgesia was reversed by induction of reactive destabilization via intrathecal injection of anisomycin and intraplantar capsaicin. Postsynaptic protein expression of (A) GluA1, (B) GluA2, (C) Shank3, (D) GKAP, and (E) PSD-95 in lumbar superficial dorsal horn tissue was quantified in animals treated with vehicle controls (Cap−, Aniso−), intrathecal anisomycin alone (Cap−, Aniso+), intraplantar capsaicin alone (Cap+, Aniso−), or treatment with both capsaicin and anisomycin (Cap+, Aniso+). (See also fig. S7, A to E). (F to J) CFA-induced hyperalgesia was similarly reversed by NI-NMDAR signaling, and postsynaptic protein in lumbar superficial dorsal horn tissue was quantified as described in (A) to (E). (See also fig. S7, F to J). (K to N) Requirement of ubiquitination and proteosome-mediated degradation to reverse hyperalgesia. Changes in mechanical withdrawal thresholds of capsaicin-induced hyperalgesia followed by a second ipsilateral intraplantar injection of capsaicin and intrathecal injection of (K) Aniso ± PYR-41 (ubiquitin-activating E1 enzyme inhibitor) and (L) 7-CK ± PYR-41. Changes in mechanical withdrawal thresholds of CFA-induced hyperalgesia followed by a second ipsilateral intraplantar injection of capsaicin and intrathecal injection of (M) Aniso ± lactacystin (protease inhibitor) and (N) MK-801 ± lactacystin. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.