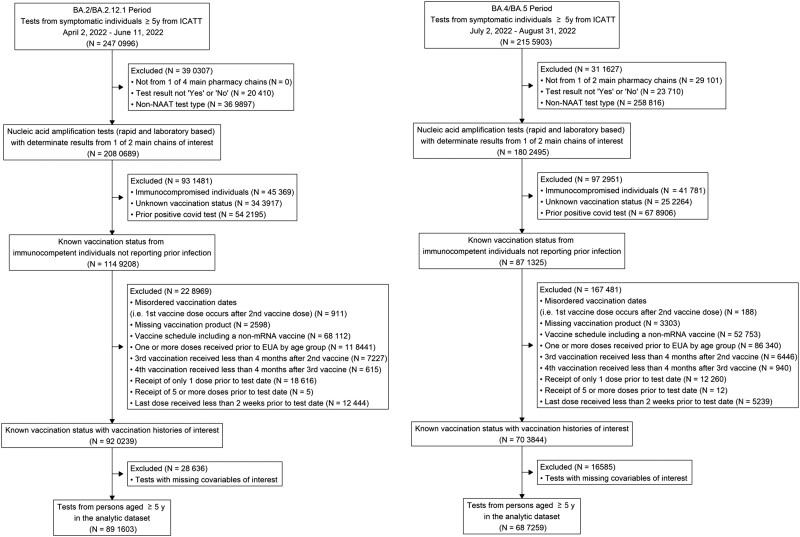
Figure 1.
Inclusion criteria for analysis of association of booster doses of an mRNA COVID-19 vaccine with symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children, adolescents, and adults during BA.2/BA.2.12.1 (April 2, 2022–June 11, 2022) and BA.4/BA.5 (July 2, 2022–August 31, 2022) predominant periods. EUA, Emergency Use Authorization; ICATT, Increasing Community Access to Testing; NAAT, nucleic acid amplification test; y, years.