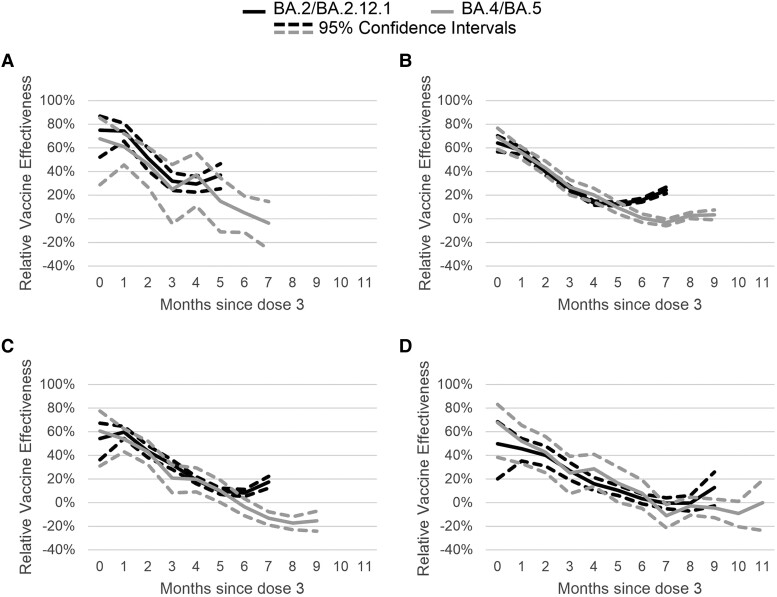
Figure 2.
Adjusted mRNA COVID-19 relative vaccine effectiveness of 3 doses compared to 2 doses against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during BA.2/BA.2.12.1 (April 2, 2022–June 11, 2022) and BA.4/BA.5 (July 2, 2022–August 31, 2022) periods by time since third dose receipt. Multivariable logistic regression models controlling for calendar day of test (continuous), age, gender, race, ethnicity, 2018 Centers for Disease Control and Prevention census tract social vulnerability index, underlying conditions (presence vs absence—included on the survey were heart conditions, high blood pressure, overweight or obesity, diabetes, current or former smoker, kidney failure or end-stage renal disease, cirrhosis of the liver, chronic lung disease [such as chronic obstructive pulmonary disease, moderate to severe asthma, cystic fibrosis, or pulmonary embolism]), individual state of residence, pharmacy chain, and average cases per 100 000 by site zip code (over the last 7 days) were used to estimate vaccine effectiveness. Tests included in the reference group were from persons receiving 2 doses and eligible for a third dose (ie, if 5 months had passed since second dose). (A) Children aged 12–15 years. (B) Young adults aged 16–49 years. (C) Adults aged 50–64 years. (D) Adults aged 65 years and older.