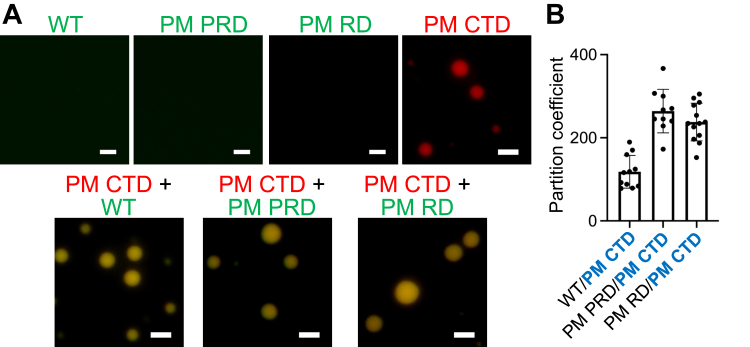
Figure 2.
Recruitment of tau variants with low LLPS propensity to droplets formed from PM CTD, the variant with high propensity for LLPS.A, (top row) representative fluorescence microscopy of the unmodified tau441 and the PM PRD and PM RD tau variants at 0.2 μM (i.e., below saturation concentration of these proteins) and the PM CTD variant at 5 μM (i.e., well above its saturation concentration); (bottom row) recruitment of the unmodified tau441 and the PM PRD and PM RD variants (0.2 μM in each case) to droplets formed by the PM CTD variant (5 μM). Unmodified tau441, the PM PRD, and PM RD variants were labeled with Alexa Fluor 488 (green), and the PM CTD variant was labeled with Alexa Fluor 594 (red). The ratio of labeled to unlabeled protein was 1:10 in each case. Scale bar represents 3 μm. B, partition coefficients for the recruitment of tau variants with low LLPS propensities (labeled in black) to the droplet phase of the PM CTD variant. These coefficients were calculated as the ratio of fluorescence intensity within the droplet and that in the dilute phase at protein concentrations as described for panel A. At least 10 droplets of each protein variant were analyzed. Error bars represent SD. Experiments were performed in 10 mM Hepes buffer (pH 7.4) containing 100 mM NaCl, 1 mM DTT, 2 mM EDTA, and 10% PEG-10. The microscopy images were obtained and fluorescence intensities within droplets measured ∼10 min after sample preparation. CTD, C-terminal domain; LLPS, liquid-liquid phase separation; PM PRD, phosphomimetic substitutions within the Pro-rich domain; PM CTD, phosphomimetic substitutions in the C-terminal domain; PM RD, phosphomimetic substitutions in the repeat domain; PRD, proline-rich domain; RD, pseudorepeat domain.