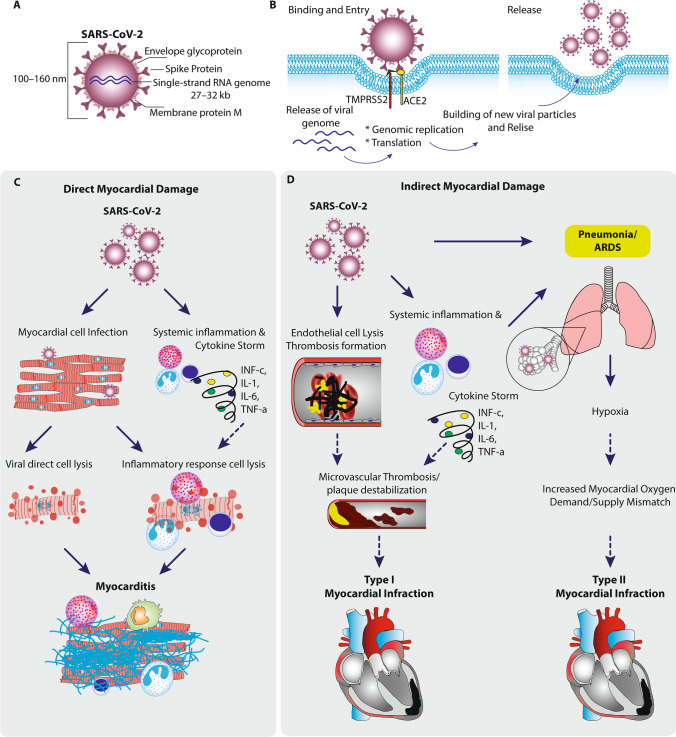
Fig. 1.
Mechanisms of myocardial damage by SARS-CoV2. A SARS-CoV-2 is a spherical viral particle consisting of three structural proteins—envelope glycoprotein, spike, and nucleocapsid protein—and a positive-sense, single-stranded RNA genome. B Infection occurs when spike protein binds to angiotensin-converting enzyme 2 (ACE2), while the transmembrane protease serine 2 (TMPRSS2) facilitates entry of the virus and the viral genome is released into the cytoplasm, followed by genomic replication/transcription and translation of viral proteins. Viral nucleocapsids are assembled in the cytoplasm, followed by budding of new particles at the membrane of the endoplasmic reticulum–Golgi intermediate compartment. Finally, viral genome and structural proteins are assembled into new viral particles and released via exocytosis. C Direct myocardial damage. Direct injury (bold arrows), through direct viral myocardial invasion and activation of inflammatory response activation. Inflammatory cells induced by SARS-CoV-2 or pro-inflammatory cytokines can cause necrosis and death of the myocardium. D Indirect injury, through interaction with ACE2, increases inflammation and endothelial dysfunction. Endothelial cell damage by SARS-CoV-2 increases the hypercoagulability state, thrombosis formation, and inflammatory response activation. Consequently, this may lead to plaque destabilization and atherothrombotic event causing type I MI. In addition, pneumonia and ARDS caused by SARS-CoV-2 and aggravated by cytokine storm exacerbate myocardial oxygen demand/supply mismatch, consequently leading to type II MI