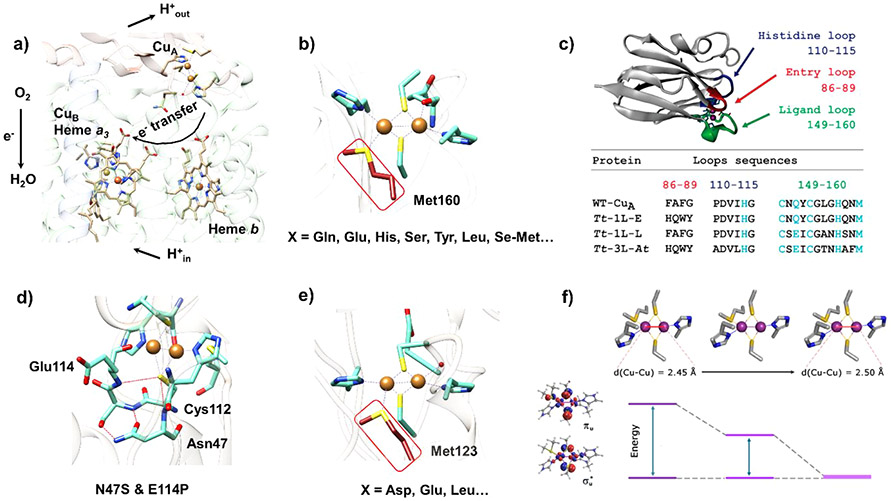
Figure 33.
(a) Schematic of the O2 reduction reaction catalyzed by heme-copper oxidase (cytochrome ba3 oxidase of T. thermophilus, PDB ID: 1XME).425 The electron transfer from CuA through heme b to heme a3 and the proton pumping pathway are shown with black arrows. (b) Mutation of the axial Met of the CuA from T. thermophilus cytochrome ba3 oxidase (PDB ID: 2CUA).346,426 (c) Loop swapping strategy to tune the CuA center.427 (d) Hydrogen bonding network surrounding the artificial CuA site in engineered azurin. (e) Mutation of the axial Met of the artificial CuA in engineered azurin (PDB ID: 1CC3).426 (f) Tuning of the πu and <u* energy gap by axial ligand mutation of T. thermophilus CuA cupredoxin.428 Parts c and f were reprinted with permission from ref 428. Copyright 2019 American Chemical Society.