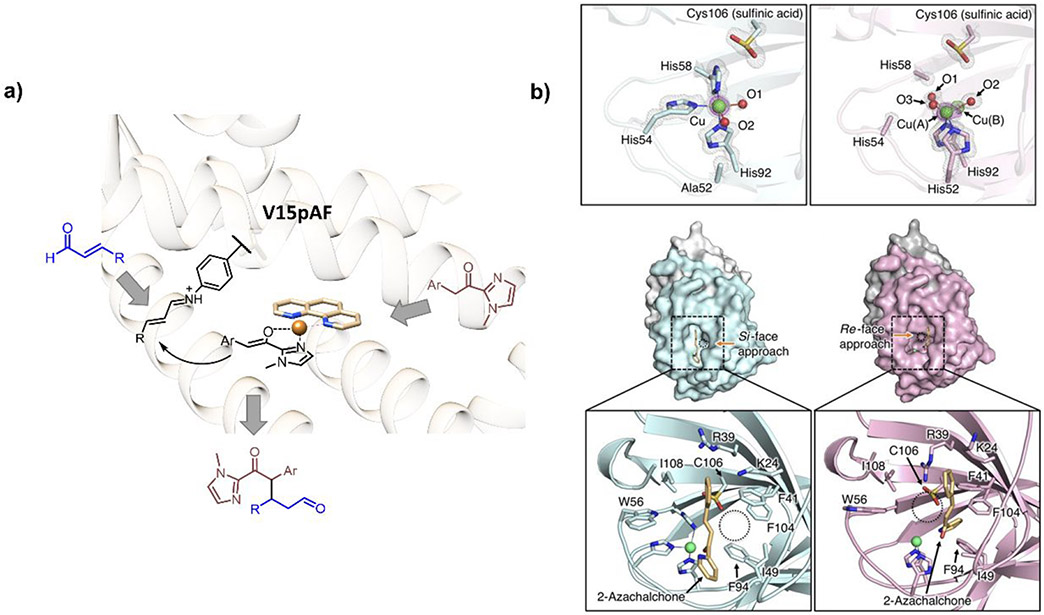
Figure 43.
(a) LmrR variant catalyzing the Michael addition reaction with an aniline moiety on an unnatural amino acid residue to activate the ketone Michael acceptor. The structure was modeled from PDB ID 6R1L,492 and the scheme was adapted from ref 464. (b) Two cupin variants with different enantioselectivities of the Michael addition reaction. The insets at the bottom demonstrate how the hydrogen bond with Cys106 changes the orientation of the substrate and thereby alters the product enantioselectivity. Reprinted with permission from refs 495 and 496. Copyright 2020 Nature Publishing Group and Wiley-VCH.