Fig. 1. CD47 + necHCs are increased in human and mouse NASH.
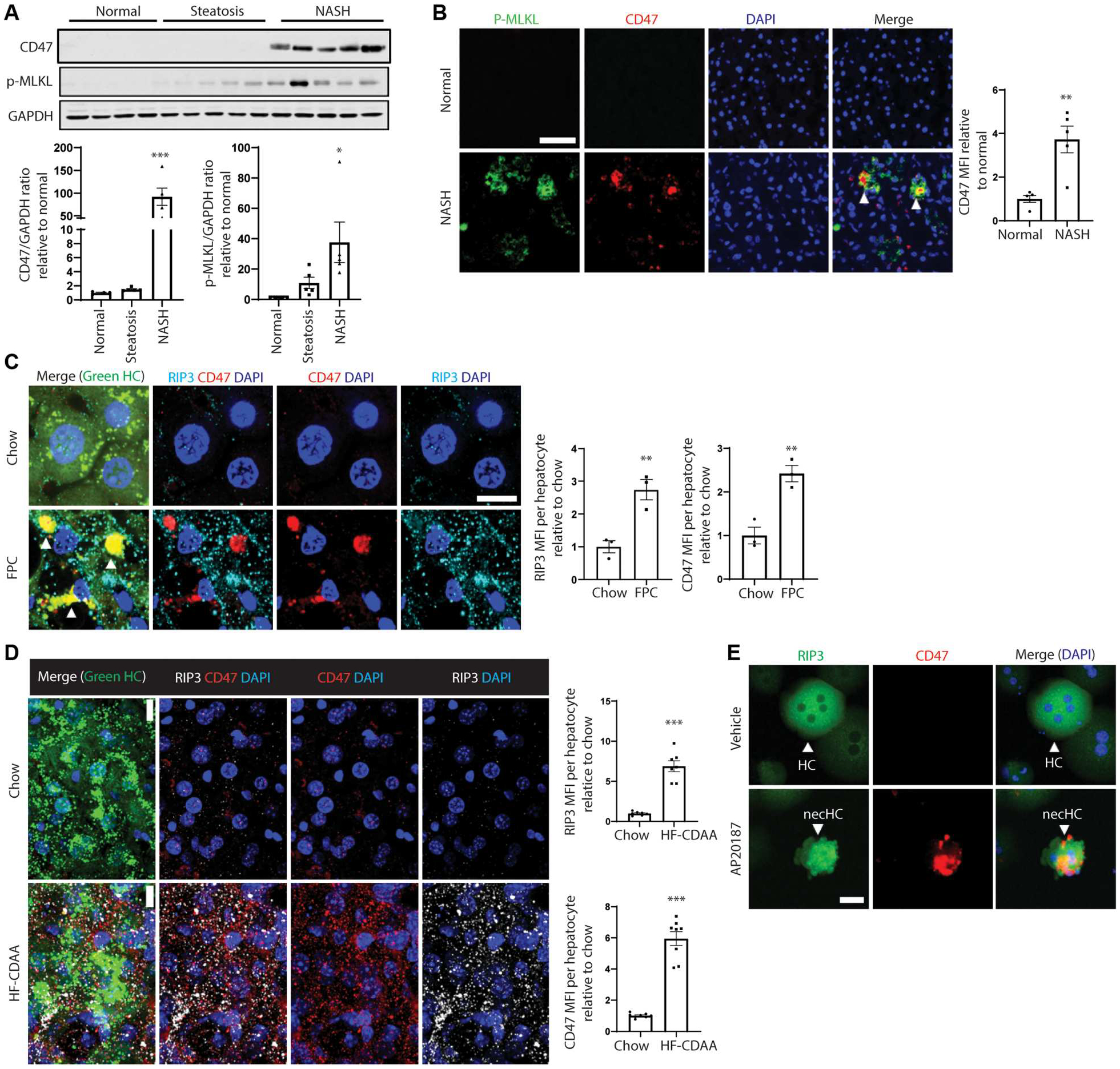
(A) Immunoblots of CD47 and p-MLKL in normal (n = 4), steatotic (n = 5), and NASH (n = 5) human livers, with data quantification (*P < 0.05; **P < 0.01 versus normal). (B) Immunofluorescence staining of human normal and NASH liver sections using anti–p-MLKL (green) and anti-CD47 (red). Arrowheads indicate p-MLKL and CD47 colocalization. Scale bar, 50 μm (**P < 0.01, n = 5 per group). (C) Male ZsGreen-inducible mice were injected with AAV8-TBG-Cre to label hepatocytes and were then fed the FPC NASH diet for 12 weeks. Liver sections (100 μm thickness) were immunostained with anti-CD47 (red) and anti-RIP3 (white). The green channel, which identifies hepatocytes, is shown in the merge image. Arrowheads indicate RIP3-CD47 colocalization. Scale bar, 10 μm. Data were quantified as mean fluorescence intensity (MFI) of CD47 and RIP3 per hepatocyte (**P < 0.01, n = 3 biological replicate hepatocytes per group). (D) Similar to (C), but the mice were fed the HF-CDAA diet for 4 weeks. Scale bar, 10 μm. ***P < 0.001, n = 7 to 8 biological replicate hepatocytes per group. (E) Primary hepatocytes isolated from AAV8-TBG-mRIP3-2xFV–injected mice were treated with vehicle control or AP20187 treatment (10 nM) to induce necroptosis ex vivo and then immunostained for RIP3 (green) and CD47 (red). Scale bar, 25 μm. necHC, necroptotic hepatocyte; HC, live hepatocyte. For all images, nuclei are stained with 4’,6-diamidino-2-phenylindole (DAPI; blue). All data are means ± SEM.
