Fig. 7. Molecular and cellular landscape of human kidney graft fibrogenesis.
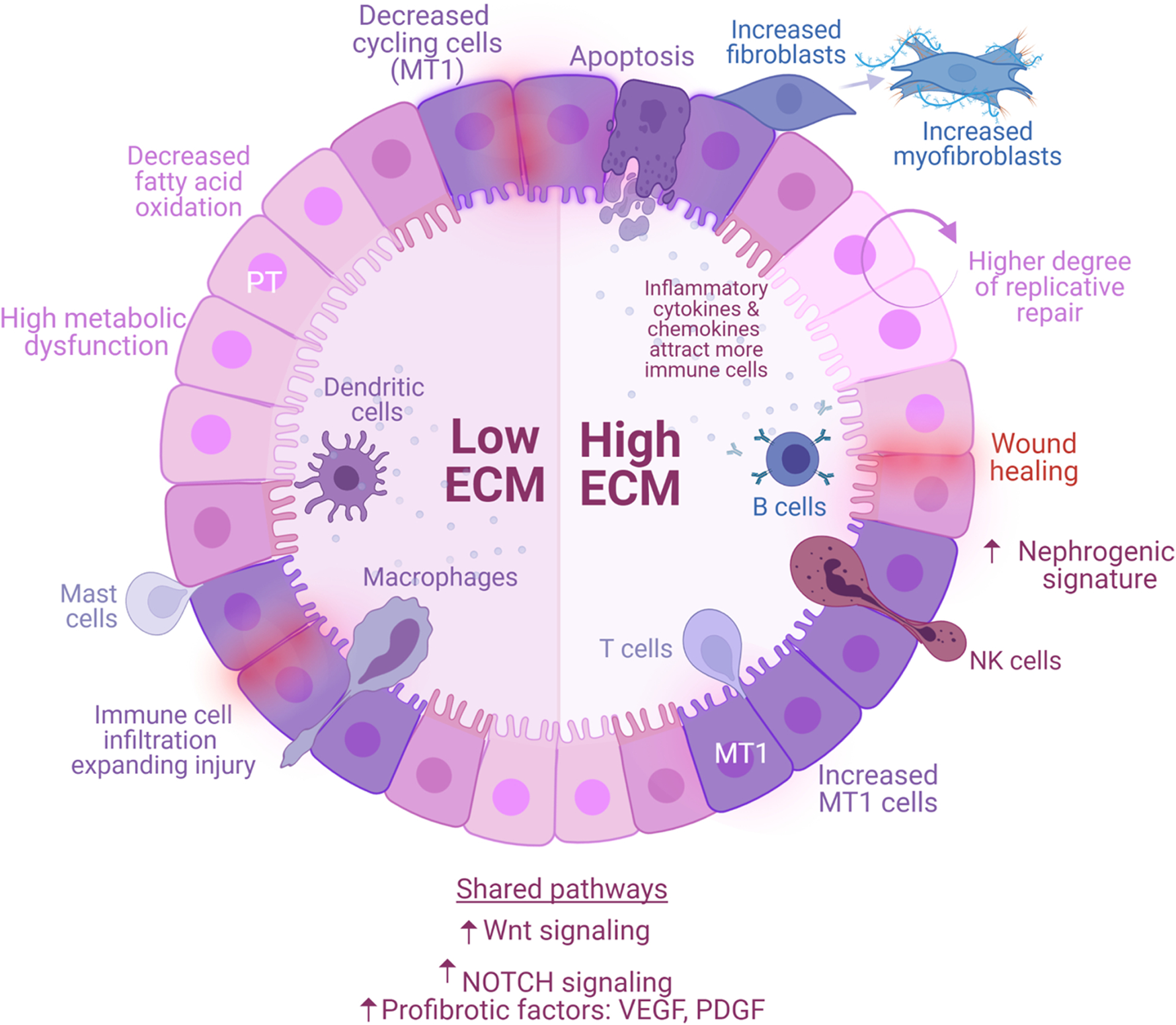
Proximal tubular (PT) epithelial cells are the first responders to kidney insults, such as ischemia reperfusion injury (early stressors) or sustained subclinical injury (late stressors). Healthy PT cells may resolve the injury and stabilize graft function (normal allografts), while injured PT cells (MT1) generate a provisional ECM that attracts inflammatory cells, facilitating their infiltration and propagating injury (fibrotic allografts). Fibrotic signaling is enhanced by secretion of growth factors (PDGF, VEGF) and upregulation of signaling pathways (NOTCH, Wnt). The processes are heterogeneous and lead to different molecular states—low ECM and high ECM. Low ECM is characterized by a decreased of cycling cells, severe PT metabolic dysfunction, and decreased apoptosis. High ECM activates maladaptive replicative repair and enters a perpetual state of tissue regeneration, scarring, and ECM accumulation. These two conditions are characterized by different proportions of immune cells. Low ECM is marked by increased dendritic (cDCs, pDCs), mast, and macrophages (MΦ1, MΦ2) cells whereas high ECM is marked by increased B, T (CD8+ T cells, NKs, and Tregs), and plasma cells. Resident macrophages and kidney cells interactions demonstrate the critical role of these cells and their cross talk in the propagation of injury. Low and high ECM are distinguishable by the secretion of different cytokines and ECM-related factors. In response to injury, MT1 cells transition into transcriptionally active fibroblasts enriched in myofibroblast markers. MT1 in high ECM was characterized by dedifferentiation and nephrogenic signatures, supporting a high degree of replicative repair, that was not observed in low ECM. Although both conditions progress to fibrosis, the severity of metabolic dysfunction of PT cells in low ECM limits repair, whereas the degree of immune cell activation in high ECM promotes a positive feedback loop inducing maladaptive repair. Interventions aimed at graft fibrogenesis likely require a more targeted approach based on the unique molecular pathways characterizing each condition.
