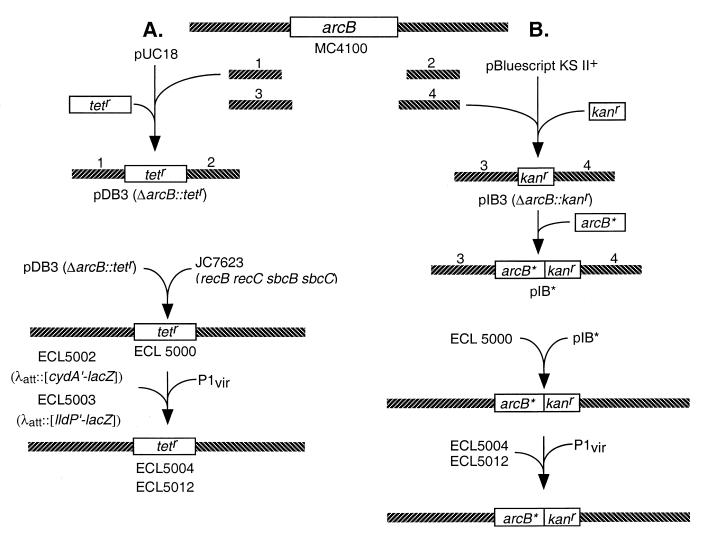
FIG. 2.
Construction of strains. (A) To construct the ΔarcB::Tetr strain, the 5′- and 3′-flanking DNA fragments of arcB (fragments 1 and 2) were prepared by PCR, using chromosomal DNA from strain MC4100 as template and, respectively, the primer pairs DAB-5N/DAB-5C and DAB-3N/DAB-3C (Table 2). The products were cloned into pUC18. A Tetr cassette isolated from pNK81 (50) was then inserted between the two arcB-flanking fragments, generating pDB3. This plasmid was transformed into strain JC7623 (36) to create ΔarcB::Tetr strain (ECL5000) by homologous recombination. The ΔarcB::Tetr allele was then P1 transduced into strain ECL5002 or ECL5003, respectively, resulting in ECL5004 and ECL5012. (B) To introduce the modified arcB sequence into the ΔarcB::Tetr strain, the 5′- and 3′-flanking DNA fragments of arcB (fragments 3 and 4) were prepared by PCR, using chromosomal DNA from strain MC4100 as template and, respectively, the primer pairs IAB-5N/IAB-5C and IAB-3N/IAB-3C (Table 2). The products were cloned into pBluescript KS II(+). A Kanr cassette, isolated from pUC4-KIXX (3), was then inserted between the two arcB-flanking fragments, generating pIB3. The 5′-flanking fragment includes the arcB promoter, ribosome-binding site, and introduced NdeI site which included the initiation codon of arcB followed by a HindIII site. Modified arcB sequences (arcB∗) were cloned into the pIB3 between the NdeI site and HindIII site, generating pIB∗. This plasmid was transformed into strain ECL5000 to replace ΔarcB::Tetr allele with arcB∗::Kanr by homologous recombination. Recombinants were selected by the Tets Kanr Amps phenotype and confirmed by the PCR. The arcB∗::Kanr was then P1 transduced into strains ECL5004 or ECL5012. Not illustrated is the construction of the reporter fusions. To construct the Φ(cydA′-lacZ) operon fusion, a 1.0-kb BamHI-EcoRI fragment of plasmid pBTKScyd1 (31) was ligated into BamHI-EcoRI-digested lacZ operon fusion vector pRS528 (42), resulting in pCAZ1. To generate the Φ(lldP′-lacZ) operon fusion, a 3.6-kb PstI-BamHI fragment of plasmid pLCT2 (9) was subcloned into pBluescript SK(−) (Stratagene), resulting in pLLD2. A 0.8-kb EcoRI-BglII fragment of pLLD2 was then ligated into the EcoRI-BamHI-digested lacZ operon fusion vector pRS415 (42), resulting in pLPZ1. The Φ(cydA′-lacZ) and Φ(lldP′-lacZ) were then transferred to the λ transducing phage λRS45 (42), yielding, respectively, λCAZ1 and λLPZ1. Lysates with high titers of λCAZ1 and λLPZ1 were used to lysogenize strain MC4100, and single lysogens were selected (28), yielding, respectively, strains ECL5001 and ECL5003. The Δfnr::Tn9 (Cmr) allele of strain JRG1728 (43) was P1 transduced into strain ECL5001 (yielding strain ECL5002) in order to avoid the transcriptional repression of Φ(cydA′-lacZ) by Fnr (8).