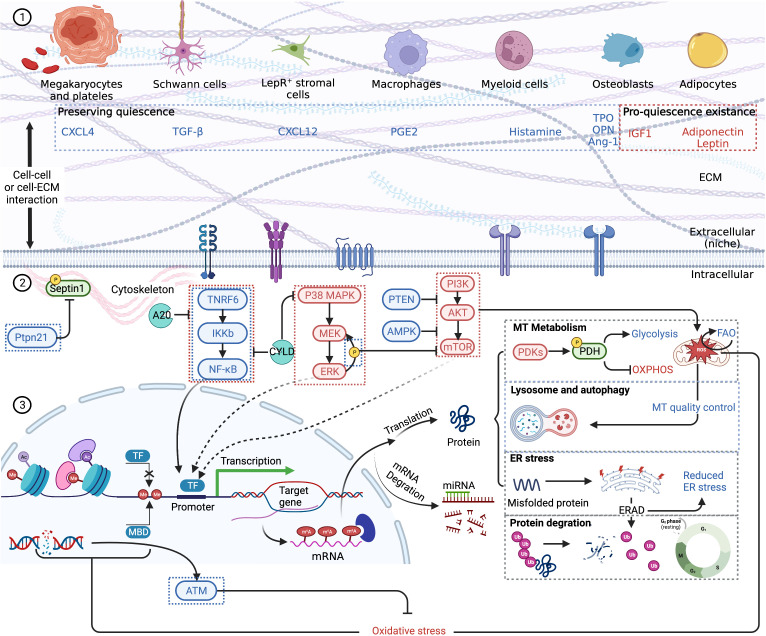
Figure 2.
A schematic summary of HSC quiescence regulation based on previous reviews (58, 59). Blue font and boxes label the factors maintaining quiescence, while red font and boxes label the factors promoting quiescence existence. (1) HSC extrinsic regulation. Niche cells and respective cytokines are pictured. HSCs receive stimulation via membrane receptors. Cell–cell interaction and cell–ECM interaction are also vital to quiescence retention. (2) HSC intrinsic regulation. NF-kB pathway, MAPK pathway, and mTOR pathway are the main signal pathways regulating HSC quiescence. Quiescent HSCs mainly depend on glycolysis for energy, and the self-renewal and maintenance of stem-cell pool rely on FAO. Lysosome and autophagy are important to clear excessively active mitochondria. Epigenetic changes, including histone and DNA modification, which influence gene transcription, are also the consequences of oxidative stress. After transcription, m6A modifications are significant epigenetic factors regulating HSC quiescence. Misfolded or unfolded protein depends on ERAD to degrade via ubiquitination. Other proteins including cell cycle proteins are also eliminated by ubiquitination, thus regulating cell dormancy and cycle activation. CXCL, CXC motif chemokine ligand; TGF, transforming growth factor; PGE, prostaglandin E; TPO, thrombopoietin; OPN, osteopontin; IGF, insulin-like growth factor; ECM, extracellular matrix; MT, mitochondrion; PDK, PDH kinases; PDH, pyruvate dehydrogenase; OXPHOS, oxidative phosphorylation; ER, endoplasmic reticulum; TF, transcription factor; MBD, methyl binding domain protein; ATM, ataxia telangiectasia mutated kinase. Created with BioRender.com.