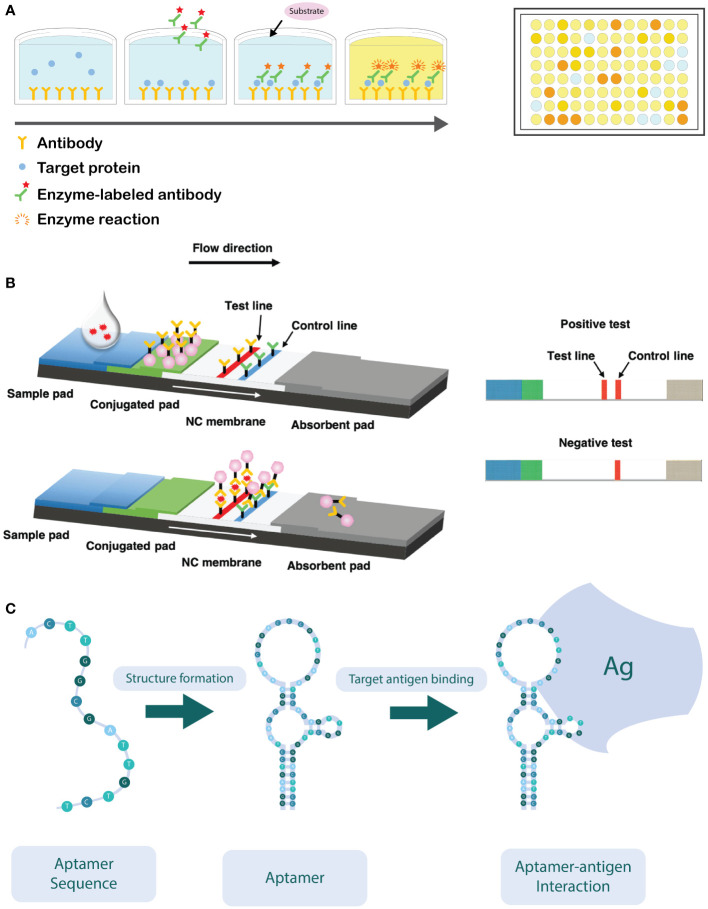
Figure 3.
(A) Schematic overview of a sandwich ELISA assay. The wells are coated with an antibody which targets the antigen. The antigen of interest in the sample is then added and binds to the capture antibody. Next, the detection antibodies are added, which target a different epitope of the antigen. The detection antibodies are labeled with an enzyme, capable of converting a colorless substrate to generate a colorimetric signal. ELISA reactions are typically performed in a 96-well plate. (B) Schematic overview of the working principle of the lateral flow immunoassay (LFIA). After application of the sample on the sample pad, it flows in the direction of the absorbent pad due to capillarity and passes through the conjugate release pad, where the labelled detector antibodies can bind to the target analyte. Next, the sample will continue to flow towards the test and control lines, where the analyte (coupled to the detector antibody) will bind to specific (secondary) antibodies immobilized in the test zone. The excess of unbound detector antibodies will flow towards the control zone, where they are bound to immobilized antibodies specific for the detector antibody. Aggregation of the labelled detector antibodies in both test and control zone can be visually observed as illustrated on the right-hand side of the figure (Adapted from Hsiao et al., 2021). (C) Schematic illustration of how the oligonucleotide sequence self-hybridizes into its functional conformation. In its functional conformation, the aptamer is able to bind to its target antigen (Ag) (Adapted from Sun et al., 2014).