Figure 6.
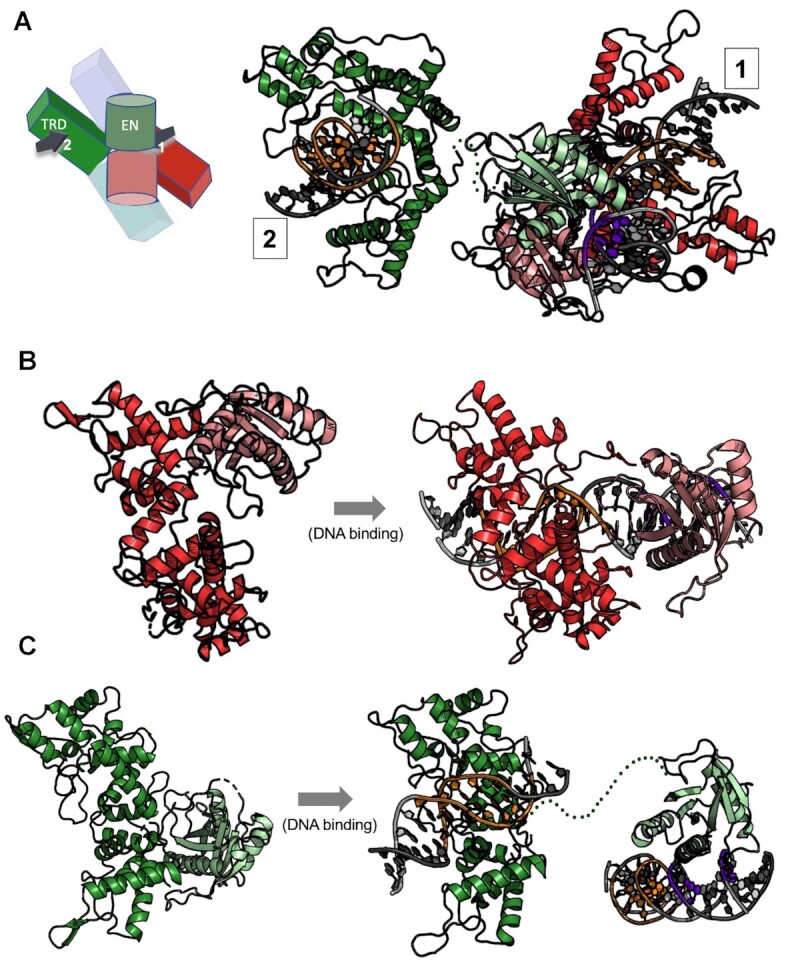
Endonuclease motions and engagement on DNA. (A) Ribbon diagram and cartoon schematic of cis and trans protein subunits (as shown in Figure 4C) that position their respective endonuclease (EN) domains on both strands and cleavage positions on a single bound DNA duplex. In the cartoon schematic the DNA duplexes are drawn as arrows and numbered according to their protein subunit (cis = 1, trans = 2) (as shown in Figure 4C). EN domains are colored in a lighter shade than the target recognition domains (TRDs). The cleavage sites of the DNA duplex are shown in purple and the target site in orange. The 16 amino acid linker between the trans-acting EN domain and its C-terminal TRD is poorly ordered in the model and are represented in the figure as a dotted green line. (B) EN domain motion of the cis-acting endonuclease domain (red subunit in Figure 4C) acting on DNA 1. The EN domain (pink) swings about 180° about the axis to align with the cleavage site of DNA 1 to cut four bases from the target site while the TRD (red) stays fixed except for slight closure of the helices to bind DNA. Panel c: EN domain motion of the trans-acting endonuclease (green subunit in Figure 4C) acting on DNA 1. The TRD (green) is bound to a separate DNA duplex (DNA 2) than the one bound by the cis-acting EN and the trans-acting EN engage for cleavage (DNA 1). The EN domain (light green) reaches across the belt of TRDs to locate the cleavage site of the DNA 1 to cleave.
