Osteosarcoma (OS) is the most common histological form of primary bone cancer in childhood cancer and young adults. At present, OS is widely investigated because of the interaction between the tumor and bone microenvironment and the effect of such interaction on OS progression and metastasis.1 The connective tissue growth factor (CTGF), also known as cellular communication network factor 2 (CCN2), is a secreted extracellular matrix-associated protein. CTGF is as active as the regulators of signaling activities of several different pathways and an orchestrator of their cross-talk.2 Therefore, we conducted experiments to investigate the effects of CTGF on OS tumor progress and the cross-talk with stromal cells in the tumor microenvironment.
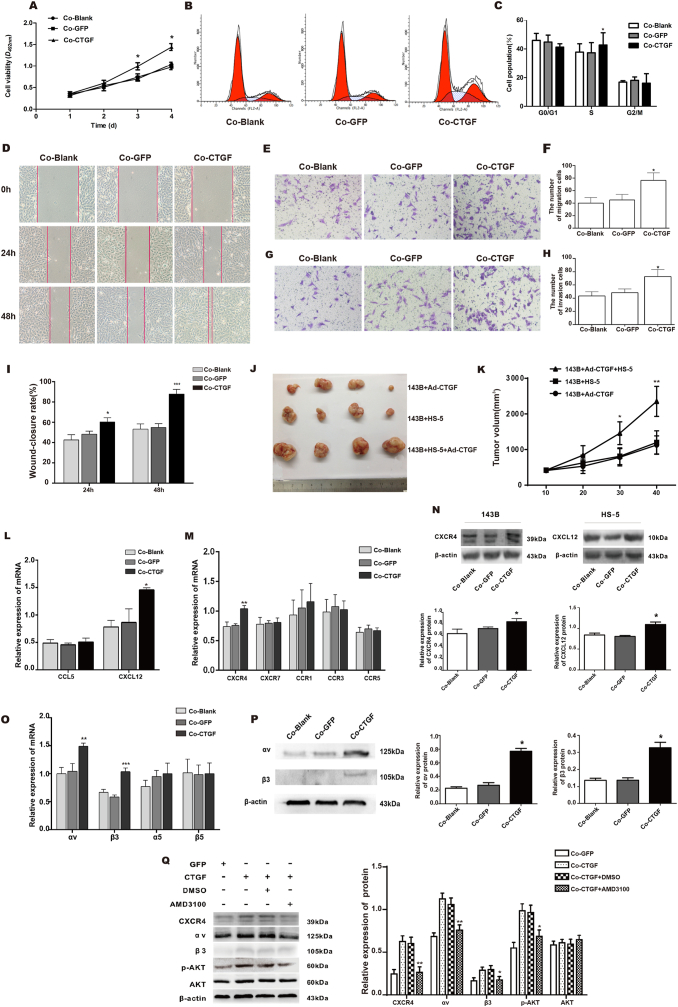
We evaluated the endogenous expression of CTGF in stromal cells and OS cell lines, including mouse embryonic mesenchymal stem cells C3H10T1/2, mouse embryo fibroblasts (MEFs), human bone marrow stromal cells HS-5, as well as human osteosarcoma cells MG-63, 143B, SaOS2, and U2OS, via quantitative reverse transcription PCR (qRT-PCR) analysis and Western blot (Fig. S1A, B). Our results showed that both 143B and HS-5 cells expressed lower levels of CTGF. Previous studies demonstrated the capacity of HS-5 to reproduce MSCs and influence tumor biology3; thus, we selected the human HS-5 and 143B cell lines for subsequent experiments. Aimed at investigating the effects of CTGF on OS cell growth in the tumor microenvironment, the 143B cells were treated with recombinant adenoviruses expressing CTGF (Ad-CTGF) or green fluorescent protein (Ad-GFP), and co-cultured with HS-5 cells for 48, 72, and 96 h. Then, the cell viability and cell cycles were examined by MTT assay and flow cytometry, respectively. As shown in Figure 1A, the cell viability of co-cultured 143B cells treated with Ad-CTGF (Co-CTGF group) was significantly promoted at 72 h and more significant increase was observed at 96 h compared with that of the co-cultured 143B cells treated with Ad-GFP group (Co-GFP group). Furthermore, the flow cytometry results showed that the percentage of S phase in Co-CTGF group was increased from 35.96% ± 3.12%–42.74% ± 4.42% (P < 0.05 vs. Co-GFP group) (Fig. 1B, C). This finding demonstrates the capacity of CTGF to promote the proliferation of 143B cells in the co-culture system. Wound-healing assay and Transwell assay were used to detect the potential capacity of migration and invasion induced by CTGF in the co-culture system. Moreover, a cell invasion assay was performed by Transwell chambers with matrigel. After co-cultivation for 48 h, the wound-closure rate (Fig. 1D, I), and the number of migration cells (Fig. 1E, F) and invasion cells (Fig. 1G, H) in the Co-CTGF group was significantly increased relative to those of the Co-GFP group and Co-Blank group. Our data show that CTGF promotes migration and invasion of 143B cells in the co-culture system in vitro.
Figure 1.
CTGF/CCN2 promotes the proliferation of human osteosarcoma cells via cross-talking with the stromal CXCL12/CXCR4-AKT-αvβ3 signaling axis in tumor microenvironment. (A) 143B cells in the Co-CTGF group were infected with Ad-CTGF for 24, 48, 72 h, and 96 h. At the end of the indicated time, cells viability was determined by MTT assays. The results represent the mean absorbance ± SD of three independent experiments (∗P < 0.05 vs. Co-GFP group). (B, C) 143B cells cultured in the Co-CTGF group for 72 h. Cell cycle distribution was analyzed by flow cytometry. Data are presented as the means values ± SD of three independent measurements (∗P < 0.05 vs. Co-GFP group). (D, I) Wound healing assay and (E, F) Transwell assay measured cell horizontal and vertical migration. 143B cells were cultured in Co-Blank, Co-GFP, Co-CTGF group for 24 and 48 h in wound healing assay and for 48 h in Transwell assay. Data are presented as mean values ± SD of three individual measurements. Magnification, × 100. (G, H) Cell invasion property was measured by Transwell invasion assay. 143B cells were cultured in each group for 48 h. Data are reported as mean values ± SD of three individual measurements. The quantification data were located right. Magnification, × 100. (∗P < 0.05 or ∗∗∗P < 0.001 vs. Co-GFP group). (J) Excised tumor tissues from the three groups. (K) Tumor growth curves of the three groups. n = 4 mice/group. Data are reported as mean ± SD. (∗P < 0.05 or ∗∗P < 0.01 vs. 143B + HS-5 group). (L, M) qRT-PCR assay determined the mRNA levels of chemokines in HS-5 cells and their special ligands in 143B cells after over 3-days’ co-culture (∗P < 0.05 or ∗∗P < 0.01 vs. Co-GFP group). (N) Western blot detected the expression levels of CXCL12 and CXCR4 proteins. Quantification data are on the lower panel. Data are reported as mean values ± SD of three individual measurements (∗P < 0.05 vs. Co-GFP group). (O) qRT-PCR assay determined the mRNA expression of integrins in 143B cells after over 3-days’ co-culture (∗∗P < 0.01 or ∗∗∗P < 0.001 vs. Co-GFP group). (P) Integrin αvβ3 protein expression of 143B cells in each group over 3-days’ co-culture were verified by Western blot. Quantification data are on the right panel (∗P < 0.05 vs. Co-GFP group). (Q) The expression levels of integrin αvβ3 and phosphorylation of AKT in 143B cells in each group after 3-days’ co-culture were detected by Western blot. Quantification data are on the right panel. Data are reported as mean values ± SD of three individual measurements (∗P < 0.05 or ∗∗P < 0.01 vs. Co-GFP group).
In vivo tumorigenicity assay is a crucial experiment for determining whether CTGF affects the property of OS cells when co-cultured with HS-5 cells. According to our data, CTGF accelerated the growth of 143B tumors mediated by HS-5 cells. No significant difference was observed among the three groups at the beginning, but the tumor volumes became palpable after 20 days. Tumor tissues excised from the three groups after 40 days was shown in Figure 1J. On the average, the group transfected with Ad-CTGF alone grew from 531 to 1276.7 mm3 and the co-culture group without Ad-CTGF grew from 623 to 1570 mm3, whereas the co-culture group with Ad-CTGF grew from 842 to 2353.3 mm3 (Fig. 1K). Our results are consistent with the in vitro observation in which CTGF markedly promoted 143B cells growth when mediated by HS-5 cells. However, the hematoxylin and eosin (H&E) staining did not show any variances in the heterogeneity of the three groups (Fig. S1C). Furthermore, tumor cells were not found in the lung tissues of the three groups.
In the cross-talk of tumor–stromal cells, chemokine/chemokine receptor loops participated in the promotion of tumor cell survival and metastasis.4 We evaluated several chemokines of HS-5 cells in each group on day 3. The mRNA level of CXCL12 was only upregulated in the Co-CTGF group (Fig. 1L). Then, we examined the expression levels of mRNA in relation to the chemokine receptors in the 143B cells and found that the mRNA expression level of CXCR4 was enhanced significantly (Fig. 1M). As for the Western blot assay, it revealed a significant increase in the protein levels of CXCR4 in the 143B cells and CXCL12 in the HS-5 cells of the Co-CTGF group (Fig. 1N). Previous research found that β1 integrin is functionally associated with enhanced tumor growth in CXCR4-overexpressed HOS cells.5 Therefore, we investigated the mRNA level of several integrins in 143B cells via qRT-PCR. αvβ3 was upregulated in the Co-CTGF group (Fig. 1O). The result of the Western blot was consistent with that of qRT-PCR (Fig. 1P). Furthermore, previous data indicate that the PI3K-AKT pathway is involved in the cross-talk of chemokines/integrin. To determine the potential mechanism of the CXCL12/CXCR4 axis in the upregulated integrin αvβ3 mediated by CTGF in the co-cultured system, we pre-treated the 143B cells with AMD3100, a CXCR4 blocker. As shown in Figure 1Q, the increased expressions of αvβ3 and phosphorylation of Akt in the CTGF-overexpressed 143B cells in the co-cultured system were inhibited after blocking CXCR4. Our results demonstrated the capacity of CTGF to enhance the expression of CXCL12/CXCR4 in the tumor-stromal intercellular, which increased the phosphorylation of AKT and then enhanced the expression of integrin αvβ3 in 143B cells.
In conclusion, we revealed the capacity of CTGF to induce the proliferation of OS cells via cross-talking with the stromal CXCL12/CXCR4-AKT-αvβ3 signaling pathway in the tumor microenvironment. Our data can provide new insights into how CTGF aggravates the pathogenesis of OS from the perspective of signal communication between tumor and stromal cells.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81102035, and 82060388), Doctor Foundation of Guizhou Provincial People's Hospital (No. GZSYBS[2019]08), Guizhou High-level Innovative Talents Project (No. QKPT[2017]5724-6), Guizhou Department and Platform Talents (No.[2017]5735-31), and Science and Technology Department of Guizhou Province (No. QKHJC[2021-396]).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.04.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yang C., Tian Y., Zhao F., et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21(19):6985. doi: 10.3390/ijms21196985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leguit R.J., Raymakers R.A.P., Hebeda K.M., Goldschmeding R. CCN2 (Cellular Communication Network factor 2) in the bone marrow microenvironment, normal and malignant hematopoiesis. J Cell Commun Signal. 2021;15(1):25–56. doi: 10.1007/s12079-020-00602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamo A., Delfino P., Gatti A., et al. HS-5 and HS-27A stromal cell lines to study bone marrow mesenchymal stromal cell-mediated support to cancer development. Front Cell Dev Biol. 2020;8:584232. doi: 10.3389/fcell.2020.584232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzenfeld P., Kossover O., Körner C., et al. Chemokine axes in breast cancer: factors of the tumor microenvironment reshape the CCR7-driven metastatic spread of luminal-A breast tumors. J Leukoc Biol. 2016;99(6):1009–1025. doi: 10.1189/jlb.3MA0815-373R. [DOI] [PubMed] [Google Scholar]
- 5.Miura K., Uniyal S., Leabu M., et al. Chemokine receptor CXCR4-beta1 integrin axis mediates tumorigenesis of osteosarcoma HOS cells. Biochem Cell Biol. 2005;83(1):36–48. doi: 10.1139/o04-106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.