FIGURE 3.
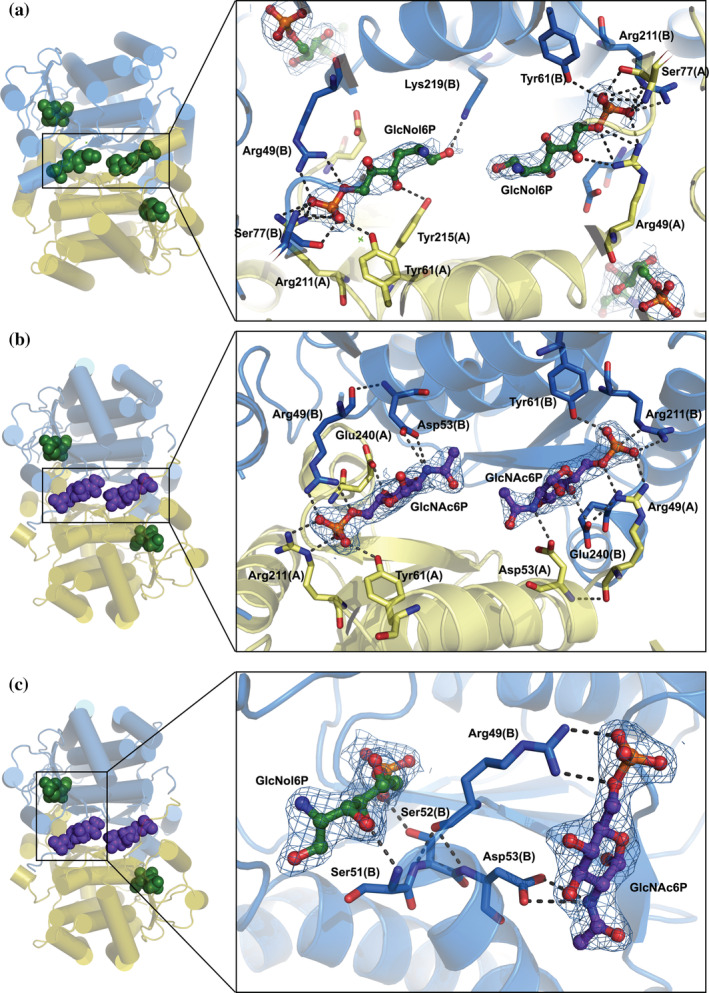
Structure of the novel allosteric site of SdNagBII. (a) The complex of SdNagBII with GlcNol6P (green) bound to both the active sites and novel allosteric site (with binding to allosteric site enlarged). (b) A complex of SdNagBII with GlcNol6P (green) at the active site and GlcNAc6P (purple) at the allosteric site (enlarged). Chains of the dimer are colored in blue and yellow, highlighting that the new allosteric site is at the interface between subunits. (c) Complex of SdNagBII with GlcNAc6P (purple) at the allosteric site and GlcNol6P (green) at the active site. Residues Arg49, Ser51, Ser52, and Asp53 communicate between the active and allosteric sites (enlarged); this segment (49–53) is unstructured in the APO form of the enzyme. For clarity, chain A is not shown in this projection. Polar interactions are indicated, and electron density maps 2Fo‐Fc contoured at 1 sigma are shown for the ligands.
