Abstract
Purpose of review
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with obesity, but is also common in individuals with a normal body mass index (BMI), who also experience the hepatic inflammation, fibrosis, and decompensated cirrhosis associated with NAFLD progression. The clinical evaluation and treatment of NAFLD in this patient population are challenging for the gastroenterologist. A better understanding of the epidemiology, natural history, and outcomes of NAFLD in individuals with normal BMI is emerging. This review examines the relationship between metabolic dysfunction and clinical characteristics associated with NAFLD in normal-weight individuals.
Recent findings
Despite a more favorable metabolic profile, normal-weight NAFLD patients exhibit metabolic dysfunction. Visceral adiposity may be a critical risk factor for NAFLD in normal-weight individuals, and waist circumference may be better than BMI for assessing metabolic risk in these patients. While screening for NAFLD is not presently recommended, recent guidelines may assist clinicians in the diagnosis, staging, and management of NAFLD in individuals with a normal BMI.
Summary
Individuals with a normal BMI likely develop NAFLD as a result of different etiologies. Subclinical metabolic dysfunction may be a key component of NAFLD in these patients, and efforts to better understand this relationship in this patient population are needed.
Keywords: Body mass index, metabolic syndrome, sarcopenic obesity, visceral adiposity, waist circumference
Introduction
Obesity is the strongest determinant of risk for nonalcoholic fatty liver disease (NAFLD) [1, 2]. Greater adiposity confers higher NAFLD risk, NAFLD prevalence increases concomitantly with body mass index (BMI) [3], and most clinical studies involve individuals belonging to overweight and obese BMI categories. This has led to the common misconception that NAFLD develops only in individuals with excess adiposity. However, NAFLD, and its progressive form, nonalcoholic steatohepatitis (NASH), are common among individuals with “normal-weight”, as defined by a BMI range of 18.5 – 24.9 kg/m2 (18.5 – 22.9 kg/m2 for Asians) [4, 5]. NAFLD prevalence in the normal BMI group ranges from 5%−34% [6] and varies by geography [7]*. The global prevalence of NAFLD in normal-weight individuals is ~11% [8], corresponding to approximately 20% of all NAFLD patients [9]. As in higher BMI groups, NAFLD prevalence is increasing in this population [10]. Although normal weight and normal BMI represent different measurements, here we use the terms interchangeably for the sake of convenience.
Individuals with NAFLD belonging to normal-weight, overweight (BMI: 25.0–29.9 kg/m2), and obese (BMI > 30 kg/m2) BMI categories share many characteristics. In some cases, NAFLD seems to represent the same clinical entity, but whose natural history, comorbidities, and mortality are amplified by the presence of excess adiposity. However, not all normal-weight individuals with NAFLD exhibit metabolic dysfunction [2] and, instead, appear to develop the disease in response to factors such as genetic susceptibility, dietary composition, hormonal status, and lifestyle behaviors [6]. In this patient population, secondary causes such as inherited disorders, lipodystrophy, drug-induced fatty liver, covert alcohol abuse, and viral infections need to be excluded before a NAFLD diagnosis can be made (Table 1). Regardless of the underlying etiology, many normal-weight NAFLD patients experience the hepatic inflammation, fibrosis, and decompensated cirrhosis associated with NAFLD progression, indicating that lower adiposity does not necessarily correspond to a milder course of the disease.
Table 1.
Potential causes of fatty liver in normal-weight individuals
| Viral | Hepatitis C, Human immunodeficiency virus (HIV) |
|---|---|
| Pharmacological | Tamoxifen, Amiodarone, some calcium channel blockers, corticosteroids, antidepressants, antipsychotics, herbal supplements |
| Heritable | Abetalipoproteinemia, lipodystrophies, cholesterol ester storage disease, Wilson’s disease, Wolman disease, PNPLA3 mutations |
| Hormonal | Hypothyroidism, estrogen deficiency |
| Metabolic | Insulin resistance, visceral adiposity, high dietary fructose intake, high dietary intake of refined carbohydrates and saturated fats |
| Other | Dietary choline deficiency, total parenteral nutrition, celiac disease, small intestinal bacterial overgrowth, gut dysbiosis, covert alcohol abuse |
Despite the risk of NAFLD in normal-weight individuals, the understanding of the pathophysiology and clinical management of this disease in this population is limited. However, recent studies have documented the epidemiology, natural history, outcomes, and mortality in normal-weight individuals with NAFLD. In this review, we summarize these findings relative to metabolic dysfunction in NAFLD patients with normal BMI. We also explore some of the conceptual and practical challenges in screening and treating NAFLD in seemingly healthy patients.
Metabolic dysfunction and NAFLD risk in individuals with a normal BMI
The majority of normal-weight NAFLD patients appear to have a lower, though still substantial, metabolic burden relative to that found within the context of higher adiposity [11]. A meta-analysis of eighty-five studies observed significantly less metabolic dysfunction in the normal BMI group compared to the overweight one [12]**. Type 2 diabetes (T2D) was present in only 19.56% of the normal-BMI NAFLD population, compared to 45.70% in the obese NAFLD group, while blood pressure and fasting blood glucose were also significantly lower in these individuals [12]. Blood pressure, fasting plasma glucose, Hb1ac, hepatic transaminases, and other metabolic measures were lower in normal-weight NAFLD participants relative to other BMI groups, yet higher than in non-NAFLD subjects, including those with obesity [13]. In this study, the cumulative incidence of metabolic syndrome was significantly higher in the female normal-BMI NAFLD group. Another study found no differences in T2D prevalence, total cholesterol, and LDL-C between nonobese and obese BMI groups, even in the presence of significantly less hypertension, lower levels of fasting plasma glucose, triglycerides, and uric acid, and higher levels of HDL-C in the former group, suggesting a similar risk for metabolic disease independent of adiposity [14]. This is consistent with findings of impaired glucose tolerance at levels similar between normal- and obese-BMI NAFLD patients [15]. In that cohort, ~30% of normal-weight NAFLD patients were diagnosed with T2D. Despite the lower burden of adverse clinical manifestations and more favorable metabolic profile, [6, 16–19], the normal-weight NAFLD population nonetheless exhibits significant metabolic dysfunction.
Given the metabolic risk to normal-weight individuals with NAFLD, the American Gastroenterological Association has recently published a Clinical Practice Update that provides Best Practice Advice on evidence-based approaches to guide clinicians in the diagnosis, staging, and management of NAFLD in lean individuals [20]**. Best Practice Advice 2 states that “Lean individuals with NAFLD should be evaluated routinely for comorbid conditions, such as T2DM, dyslipidemia, and hypertension.” This best practice advice is based on data indicating that the normal-weight NAFLD population has a similar or higher prevalence of multiple metabolic and cardiovascular risk factors and cardiovascular events than those with overweight and obesity. The practice warns of the risk for progressive liver disease in normal-weight individuals, in whom, NAFLD should not be considered benign. All normal-weight NAFLD patients should therefore undergo noninvasive tests for risk stratification, the results of which may classify those at highest risk for progressive disease. Regardless of the progressive nature of the disease, general population screening for NAFLD in individuals with normal weight is not recommended in the absence of co-morbidities. However, with findings of elevated liver biochemical tests, multiple cardiometabolic disease risk factors, or incidentally diagnosed hepatic steatosis, the differential diagnosis should include NAFLD irrespective of BMI.
The lack of weight dependency in NAFLD may reflect the inadequacy of BMI as a measure of body composition [21]. In one study, waist circumference and serum triglyceride levels were the two most important variables associated with NAFLD in normal-weight Asians [22], while another found that normal-weight patients with a greater waist circumference (>88 cm for women, >102 cm for men) had a higher risk of T2D, liver fibrosis, and carotid plaque compared with non-lean NAFLD patients [22]. However, even with a normal waist circumference, normal-weight NAFLD patients had a significantly higher visceral adiposity index and risk of diabetes compared to non-lean patients with NAFLD [23]. Thus, it may be that visceral adiposity, not overall adiposity, is the key risk factor for NAFLD in the normal-weight population [24]**. An analysis of NHANES data (2017–2018 survey) identified waist circumference and total abdominal fat area as the best predictors of NAFLD in men, while in women, the best predictor was visceral fat [25]**. Visceral adipose tissue was higher, increased with fibrosis stage, and was associated with multi-organ insulin resistance in NAFLD patients, including those who were lean and nondiabetic [26]*. Visceral adipose tissue has also been correlated with hepatic steatosis [27]. Because it better reflects visceral adiposity, waist circumference may be a more appropriate tool than BMI for metabolic risk assessment in normal-weight NAFLD patients [22, 28]*.
In contrast to visceral adipose tissue, the association of other sites of adiposity with NAFLD has not been well characterized. In a study of more than 700 individuals from Taiwan, patients with mild to moderate NAFLD had greater volumes of pericardial and thoracic peri-aortic adipose tissue than those with normal liver findings [29]*. These two adipose depots were independently associated with NAFLD, even after adjustment for age, sex, cholesterol, and triglycerides.
Low skeletal muscle mass may also be more of a risk factor for NAFLD in normal-weight individuals than for those with overweight or obesity [16]. The association between skeletal muscle mass to visceral fat ratio and incident NAFLD, as well as more severe disease, was stronger in individuals without obesity, reflecting the importance of skeletal muscle as a major site for glucose disposal [30]. Mechanistically, low skeletal muscle mass would promote increased lipolysis in adipose tissue, resulting in higher free fatty acid flux into the liver and increased risk of hepatic fat accumulation.
Clinical characteristics of NAFLD in normal-weight individuals
NAFLD in normal-weight individuals has been shown to be independently associated with younger age, female sex, and a lower prevalence of insulin resistance and hypercholesterolemia, relative to NAFLD in overweight or obesity [31]. NAFLD in the presence of a normal BMI may also be more common among Asian- and African Americans [32]. Normal-weight individuals were more likely to develop NAFLD if they had overweight (relative risk [RR]: 2.24, 95%CI 1.42 to 3.54) or obesity (RR: 2.46, 95%CI: 1.40 to 4.31) ten years prior, implying a residual long-term effect of previous adiposity [33]*.
Some studies have indicated that normal-weight individuals may exhibit a less severe presentation of disease [34], lower likelihood of NASH [35], and lower rates of cirrhosis [17, 18] than higher BMI groups. However, many other studies have reported a similar prevalence of NASH and severity of inflammation and fibrosis between lean and non-lean NAFLD patients [34–39]. In NHANES III participants, individuals with normal BMI had the same risk of cirrhosis and decompensation, malignancy, and cardiovascular events as those in the overweight and obese categories [32]. Other studies observed a greater rate of cirrhosis in normal-weight individuals compared to higher BMI groups (11% vs ~3%) [39] and an increased risk for severe liver disease [40]. Such findings suggest that leaner individuals may experience a faster rate of fibrosis progression than patients with a higher BMI.
NAFLD may also increase mortality in the normal-weight population. Among normal-weight individuals in NHANES III, the weighted, unadjusted all-cause mortality was significantly higher in those with NAFLD than those without (40.9% vs. 17.9%, P <0.001) over a median follow-up period of 17.8 years [41]. In this study, the unadjusted hazard ratio (HR) for all-cause mortality was 2.44 (95% CI 1.77–3.37) in normal-weight NAFLD patients, who also had a 238% increased risk for cardiovascular mortality. In Austrian NAFLD patients (38 lean, 165 overweight, and 93 obese), lean patients had a significantly higher mortality rate (11%) from liver-related causes compared to the overweight (4%) and obese groups (4%) [39]. In American NAFLD patients, the normal BMI group had a higher risk of death than the high BMI group [32]. Similar results reported a higher 15-year cumulative all-cause mortality (76.3%) in lean NAFLD patients compared to those with overweight (51.7%) and obesity (27.2%), as well as individuals without NAFLD (20.7%) [42]. However, other work found a similar rate of overall survival in normal and high BMI groups, although the median follow-up period in that study was only 7.6 years [34], which may have been insufficient to detect significant differences.
The most severe complication of NAFLD, cirrhosis, has emerged as the second leading indication for liver transplantation. In a study of 82 obese and 29 lean NAFLD patients who underwent liver transplant, the lean group had worse renal function and more severe ascites than the obese group [43]*, suggesting that patients with obesity had better compensated cirrhosis, perhaps due to higher nutrient intake. Patients with obesity also had higher pre-transplant waiting list survival, lower risk of organ loss within 90 days post-surgery, a lower post-transplant death rate, a higher graft survival rate, and higher overall survival rate through five years compared to lean patients. These data are consistent with another study reporting a higher rate of removal from a waiting list and higher graft failure and all-cause mortality in normal-weight patients undergoing liver transplantation relative to those with obesity [44]. These findings are important and clearly demonstrate the need for more evidence to adequately guide the clinical management of lean patients with NAFLD cirrhosis.
Screening and clinical management of NAFLD in normal-weight individuals
As noted, NAFLD screening is not recommended for normal-weight individuals. Some specialists in the United States suggest screening individuals at risk of developing liver disease, such as those older than 50 years and with T2D or metabolic syndrome, using liver function tests and abdominal ultrasound in a primary care setting and imaging or prediction algorithms to assess the presence of fibrosis and subsequent diagnosis of NASH and staging of fibrosis [45]. European and Asian guidelines acknowledge the significance of NAFLD in normal-weight individuals, especially in those of Asian ancestry or who exhibit components of metabolic syndrome [46–48]. Knowledge of disease etiology, screening, detection methods, and consensus guidelines is becoming increasingly important for adequate clinical care of NAFLD patients, independent of BMI category, especially for primary care physicians.
The clinical management of NAFLD in normal-weight individuals is not well defined. In many cases, it may not seem like there is excess weight to lose or risk-increasing lifestyle factors to modify, and the use of pharmacological agents is poorly understood in this patient population. For gastroenterologists and hepatologists who manage NAFLD patients, an understanding of risk factors, genetic susceptibility, and risk for progressive disease specific to normal-weight individuals is necessary. Despite a normal BMI, lean individuals with NAFLD appear to respond favorably to diet and lifestyle modifications typically utilized in the treatment of obesity-related NAFLD (Fig 1). For example, a 5% weight reduction in lean individuals yielded significant improvements in ALT and AST levels, hepatic steatosis, and liver stiffness and resolved NAFLD in 57% of participants [49]. Sinn et al [50] also reported a positive association between weight reduction and fatty liver resolution in lean NAFLD patients, with a fully adjusted hazard ratio of 2.7. Thus, lifestyle modifications and weight loss may be sufficient to resolve NAFLD in some normal-weight individuals. In NAFLD patients with obesity, improvements in liver fat metabolism occur in response to a ketogenic [51] and low carbohydrate [52] diets and current EASL guidelines recommend the Mediterranean diet as a dietary treatment for NAFLD [46]. While clinical trials involving dietary interventions in NAFLD have been limited to individuals with overweight/obesity, it is likely that similar benefits of these diets may benefit normal-weight NAFLD patients. However, more research to determine optimal macronutrient composition at different stages of disease and whether reductions in central obesity through a nutritional regimen and exercise are appropriate therapeutic approaches in this patient population.
Figure 1. Possible strategies for reducing liver fat in individuals with normal BMI.
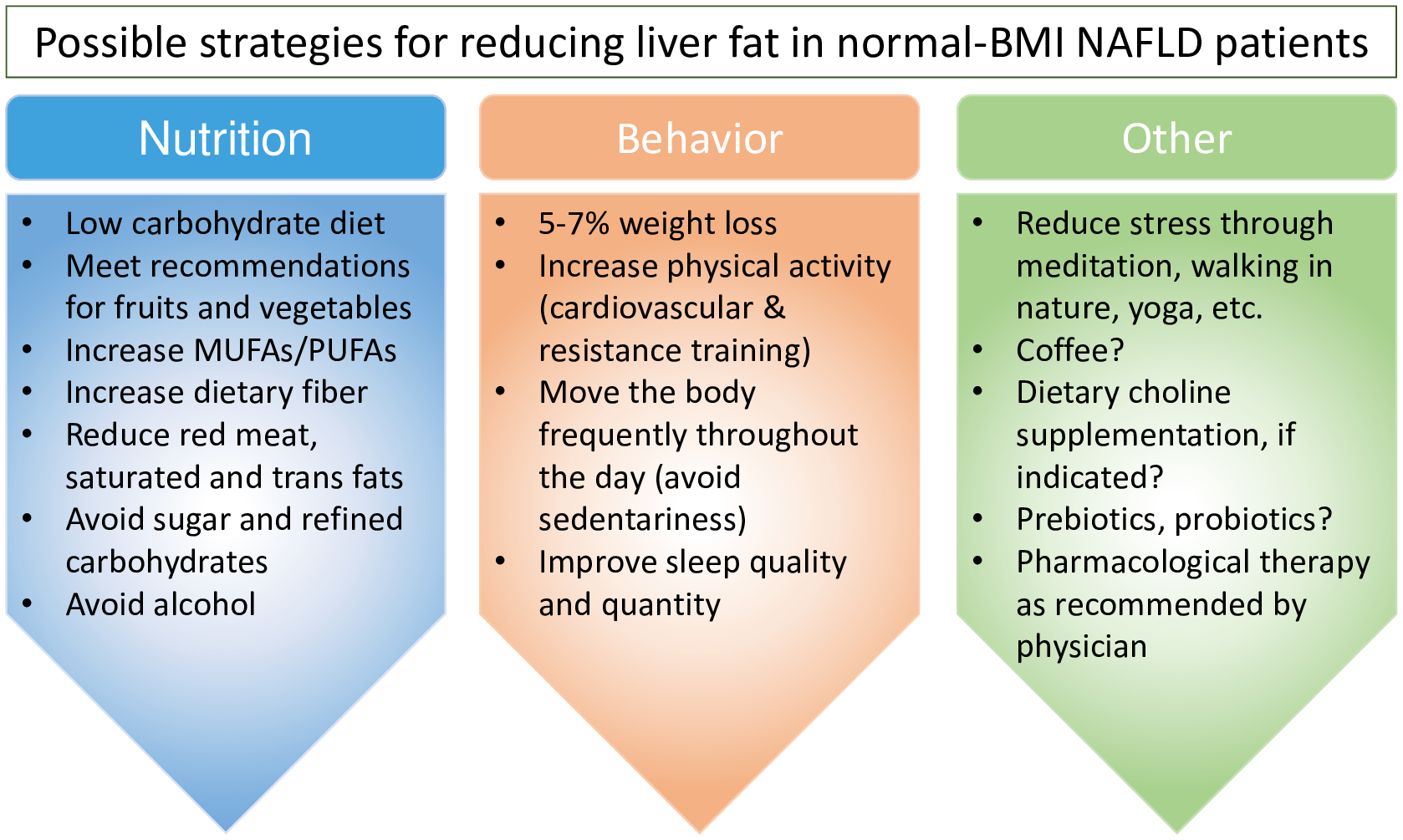
Strategies used in the treatment of NAFLD in individuals with higher BMI [56, 57] may also apply to those whose BMI is in the normal range. Recommendations followed by a question mark are speculations of the authors, but are supported by emerging evidence [6].
Conclusions
NAFLD in normal-weight individuals likely develops due to diverse underlying pathologies. However, for a significant number of individuals, excessive hepatic fat accumulation and metabolic dysfunction are tightly linked, although it is not yet clear whether one causes the other or if they develop concomitantly in response to specific environmental stimuli, such as high intake of refined carbohydrates. Characterization of NAFLD in normal-weight individuals, including identification of potential subtypes within this population, and identification of factors that modulate disease risk in the absence of clinically significant metabolic dysfunction are critical. Elucidation of disease etiology specific to each normal-weight NAFLD patient may provide the foundation upon which precision strategies for clinical management can be devised.
Measures of adiposity that are more accurate than BMI are necessary to reduce the burden of NAFLD in the normal-weight population. Some have suggested an adiposity-based constellation of chronic disease-relevant variables comprising etiology, degree of adiposity, and health risks [53] or use of the term “sarcopenic obesity” [28] as an alternative to BMI. It is now apparent that a body weight that falls within the normal BMI range does not inevitably indicate metabolic health [53], as a quarter of all adults with a normal BMI in the United States exhibit an abnormal metabolic profile [54]. Furthermore, we believe that a comprehensive re-evaluation of BMI across racial/ethnic groups may be clinically valuable, similar to a recent study of T2D incidence. In 1.33 million White, 75,000 South Asian, 50,000 Black, 10,000 Chinese, and 2500 Arab participants, BMI cutoffs for age- and sex-adjusted T2D incidence were 23.9 kg/m2 for South Asian, 28.1 kg/m2 for Black, 26.9 kg/m2 for Chinese, and 26.6 kg/m2 for Arab populations relative to a BMI of 30.0 kg/m2 for Whites [55].
In the United States, most individuals with NAFLD are diagnosed and treated in the primary care setting. Awareness of the potential health risks associated with NAFLD in normal-weight individuals and management of these factors in this population is indispensable for practitioners. Early detection, combined with efforts to resolve NAFLD through patient-specific lifestyle modifications or clinical interventions, remains the only way to prevent the progression to NASH in normal-weight individuals.
Key Points.
The development and progression of NAFLD is not uncommon in individuals with a normal BMI.
Despite a lower burden of adverse clinical manifestations and more favorable metabolic profile, the normal-weight NAFLD population exhibits significant metabolic dysfunction.
The clinical management of NAFLD in normal-weight individuals is not well defined.
In some normal-weight individuals, lifestyle modifications and weight loss may be sufficient to resolve NAFLD, although more research to determine whether reductions in central obesity through a nutritional regimen and exercise are appropriate therapeutic approaches in this patient population.
Financial Support and Sponsorship:
NIDDK R01 DK120890, R01 DK107735
Footnotes
Conflicts of Interest: None
References
- 1.Fabbrini E, Sullivan S, and Klein S, Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010; 51: 679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz Y and Younossi ZM, Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis 2014; 18: 19–31. [DOI] [PubMed] [Google Scholar]
- 3.Fan R, Wang J, and Du J, Association between body mass index and fatty liver risk: A dose-response analysis. Sci Rep 2018; 8: 15273–15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Expert Consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–63. [DOI] [PubMed] [Google Scholar]
- 5.Weir CB and Jan A, BMI classification percentile and cut off points, in Statpearls. 2022: Treasure Island (FL). [PubMed] [Google Scholar]
- 6.DiStefano JK and Gerhard GS, NAFLD in normal weight individuals. Diabetol Metab Syndr 2022; 14: 45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Yip TC, Vilar-Gomez E, Petta S, et al. , Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatology 2022. Online article ahead of print [DOI] [PubMed] [Google Scholar]; This review summarizes NAFLD across geographies and ethnicities, the role of genetic variants for NAFLD, characteristics of special populations including lean, children, type 2 diabetes, liver and non-liver-related outcomes, and economic aspects.
- 8.Henry L, Paik J, and Younossi ZM, Review article: The epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther 2022; 56: 942–956. [DOI] [PubMed] [Google Scholar]
- 9.Ye Q, Zou B, Yeo YH, et al. , Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 739–752. [DOI] [PubMed] [Google Scholar]
- 10.Ge X, Zheng L, Wang M, et al. , Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 2020; 10: e036663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary NS, Saraf N, Saigal S, et al. , Nonalcoholic fatty liver in lean individuals: Clinicobiochemical correlates of histopathology in 157 liver biopsies from healthy liver donors. J Clin Exp Hepatol 2021; 11: 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Tang A, Ng CH, Phang PH, et al. , Comparative burden of metabolic dysfunction in lean NAFLD vs. Non-lean NAFLD - a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022. Online article ahead of print [DOI] [PubMed] [Google Scholar]; In this large meta-analysis, metabolic dysfunction in NAFLD was assessed in lean individuals and compared with non-NAFLD and NAFLD in overweight and obese group
- 13.Wang W, Ren J, Zhou W, et al. , Lean non-alcoholic fatty liver disease (lean-nafld) and the development of metabolic syndrome: A retrospective study. Sci Rep 2022; 12: 10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Wang Q, Sun Y, et al. , The prevalence of lean/nonobese nonalcoholic fatty liver disease: A systematic review and meta-analysis. J Clin Gastroenterol 2020; 54: 378–387. [DOI] [PubMed] [Google Scholar]
- 15.Feldman A, Eder SK, Felder TK, et al. , Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol 2017; 112: 102–110. [DOI] [PubMed] [Google Scholar]
- 16.Byeon JH, Kang MK, and Kim MC, Association of low skeletal muscle mass with the phenotype of lean non-alcoholic fatty liver disease. Healthcare (Basel) 2022; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trifan A, Rotaru A, Stafie R, et al. , Clinical and laboratory characteristics of normal weight and obese individuals with non-alcoholic fatty liver disease. Diagnostics (Basel) 2022; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg EM, Trinh HN, Firpi RJ, et al. , Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol 2021; 19: 996–1008 e6. [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Ye J, Shao C, et al. , Varied relationship of lipid and lipoprotein profiles to liver fat content in phenotypes of metabolic associated fatty liver disease. Front Endocrinol (Lausanne) 2021; 12: 691556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Long MT, Noureddin M, and Lim JK, Aga clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology 2022; 163: 764–774 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an expert review that was commissioned and approved by the American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee and the AGA Governing Board that issued 15 Best Practice Advice guideslines to assist clinicians in evidence-based approaches to the diagnosis, staging, and management of NAFLD in lean individuals.
- 21.Ren TY and Fan JG, What are the clinical settings and outcomes of lean NAFLD? Nat Rev Gastroenterol Hepatol 2021; 18: 289–290. [DOI] [PubMed] [Google Scholar]
- 22.Xu R, Pan J, Zhou W, et al. , Recent advances in lean NAFLD. Biomed Pharmacother 2022; 153: 113331. [DOI] [PubMed] [Google Scholar]
- 23.Feng RN, Du SS, Wang C, et al. , Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight chinese population. World J Gastroenterol 2014; 20: 17932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Lee S, Kim KW, Lee J, et al. , Visceral adiposity as a risk factor for lean non-alcoholic fatty liver disease in potential living liver donors. J Gastroenterol Hepatol 2021; 36: 3212–3218. [DOI] [PubMed] [Google Scholar]; This study showed visceral adiposity may be a risk factor for lean NAFLD in lean living donor candidates who had undergone liver biopsy as part of the screening process.
- **25.Jones GS, Graubard BI, Alvarez CS, and McGlynn KA, Prediction of nonalcoholic fatty liver disease using anthropometry and body fat measures by sex and race/ethnicity in the united states. Obesity (Silver Spring) 2022; 30: 1760–1765. [DOI] [PubMed] [Google Scholar]; This was an analysis that included 1,404 participants from the 2017–2018 NHANES (National Health and Nutrition Examination Survey) data. Area under the curve (AUC) analysis found that among men, the best predictors of NAFLD were total abdominal fat area and waist circumference but among women the best predictor was visceral fat.
- *26.Saponaro C, Sabatini S, Gaggini M, et al. , Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int 2022; 42: 2418–2427. [DOI] [PubMed] [Google Scholar]; This study found that visceral fat (VF) was higher in lean and non-lean NAFLD and associated with fibrosis stage, insulin resistance in liver, muscle mass and other adipose tissue depots, decreased adiponectin, and increased lipolysis.
- 27.Brand T, van den Munckhof ICL, van der Graaf M, et al. , Superficial vs deep subcutaneous adipose tissue: Sex-specific associations with hepatic steatosis and metabolic traits. J Clin Endocrinol Metab 2021; 106: e3881–e3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Nadolsky KZ, Use of the term, “lean,” for NAFLD in individuals with low BMI. Gastroenterology 2022. Online article ahead of print. [DOI] [PubMed] [Google Scholar]; This letter brings up semantic issues using the term “lean”.
- *29.Lee CW, Yun CH, Huang WH, et al. , The association of pericardial fat and peri-aortic fat with severity of nonalcoholic fatty liver disease. Sci Rep 2022; 12: 14014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Levels of pericardial and thoracic peri-aortic adipose tissue were found to be independently associated with NAFLD after counting for sex, age, cholesterol, triglyceride, and other cardiometabolic risk factors.
- 30.Cho Y, Chang Y, Ryu S, et al. , Skeletal muscle mass to visceral fat area ratio as a predictor of nafld in lean and overweight men and women with effect modification by sex. Hepatol Commun 2022; 6: 2238–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younossi ZM, Stepanova M, Negro F, et al. , Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012; 91: 319–27. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed OT, Gidener T, Mara KC, et al. , Natural history of nonalcoholic fatty liver disease with normal body mass index: A population-based study. Clin Gastroenterol Hepatol 2021; 20: 1374–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Nah BKY, Ng CH, Chan KE, et al. , Historical changes in weight classes and the influence of nafld prevalence: A population analysis of 34,486 individuals. Int J Environ Res Public Health 2022; 19: 9935–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, 34,486 individuals from the United States National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 were analysed for historical weight changes 10 years apart. Overweight or obese individuals at both timepoints were more likely to develop NAFLD those who were lean at both time points.
- 34.Younes R, Govaere O, Petta S, et al. , Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: Time for reappraisal of BMI-driven approach? Gut 2021; 71: 382–390. [DOI] [PubMed] [Google Scholar]
- 35.Sookoian S and Pirola CJ, Systematic review with meta-analysis: The significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2018; 47: 16–25. [DOI] [PubMed] [Google Scholar]
- 36.Margariti A, Deutsch M, Manolakopoulos S, et al. , The severity of histologic liver lesions is independent of body mass index in patients with nonalcoholic fatty liver disease. J Clin Gastroenterol 2013; 47: 280–286. [DOI] [PubMed] [Google Scholar]
- 37.Fracanzani AL, Petta S, Lombardi R, et al. , Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin Gastroenterol Hepatol 2017; 15: 1604–1611. [DOI] [PubMed] [Google Scholar]
- 38.Denkmayr L, Feldman A, Stechemesser L, et al. , Lean patients with non-alcoholic fatty liver disease have a severe histological phenotype similar to obese patients. J Clin Med 2018; 17: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman A, Wernly B, Strebinger G, et al. , Liver-related mortality is increased in lean subjects with non- alcoholic fatty liver disease compared to overweight and obese subjects. J Gastrointestin Liver Dis 2021; 30: 366–373. [DOI] [PubMed] [Google Scholar]
- 40.Hagstrom H, Nasr P, Ekstedt M, et al. , Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun 2018; 2: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golabi P, Paik J, Fukui N, et al. , Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes 2019; 37: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou B, Yeo YH, Nguyen VH, et al. , Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the united states, 1999–2016. J Intern Med 2020; 288: 139–151. [DOI] [PubMed] [Google Scholar]
- *43.Qazi-Arisar FA, Uchila R, Chen C, et al. , Divergent trajectories of lean vs obese non-alcoholic steatohepatitis patients from listing to post-transplant: A retrospective cohort study. World J Gastroenterol 2022; 28: 3218–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]; A single institution study retrospectively reviewed all adult NASH patients listed for liver transplant. Lean NASH patients were found to have a substantially higher risk of graft loss within 90 d of transplant, higher death rate post transplant and worse 1– 3- and 5-year graft survival.
- 44.Ochoa-Allemant P, Trivedi HD, Saberi B, et al. , Waitlist and posttransplantation outcomes of lean individuals with nonalcoholic fatty liver disease. Liver Transpl 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Pandyarajan V, Gish RG, Alkhouri N, and Noureddin M, Screening for nonalcoholic fatty liver disease in the primary care clinic. Gastroenterol Hepatol (N Y) 2019; 15: 357–365. [PMC free article] [PubMed] [Google Scholar]
- 46.European Association for the Study of the Liver, European Association for the Study of Diabetes, Disease and European Association for the Study of Obesity, EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–402. [DOI] [PubMed] [Google Scholar]
- 47.Wong VW, Chan WK, Chitturi S, et al. , Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol 2018; 33: 70–85. [DOI] [PubMed] [Google Scholar]
- 48.Fan JG, Wei L, Zhuang H, et al. , Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, china). J Dig Dis 2019; 20: 163–173. [DOI] [PubMed] [Google Scholar]
- 49.Hamurcu Varol P, Kaya E, Alphan E, and Yilmaz Y, Role of intensive dietary and lifestyle interventions in the treatment of lean nonalcoholic fatty liver disease patients. Eur J Gastroenterol Hepatol 2020; 32: 1352–1357. [DOI] [PubMed] [Google Scholar]
- 50.Sinha M, Tripathi T, Rai P, and Gupta SK, Authors’ response. Am J Orthod Dentofacial Orthop 2017; 151: 836–837. [DOI] [PubMed] [Google Scholar]
- 51.Luukkonen PK, Dufour S, Lyu K, et al. , Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2020; 117: 7347–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mardinoglu A, Wu H, Bjornson E, et al. , An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab 2018; 27: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golubic R, Barber TM, and Caleyachetty R, Obesity definition for personalised treatment of type 2 diabetes. Lancet 2022; 399: 2189. [DOI] [PubMed] [Google Scholar]
- 54.Wildman RP, Muntner P, Reynolds K, et al. , The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008; 168: 1617–24. [DOI] [PubMed] [Google Scholar]
- 55.Caleyachetty R, Barber TM, Mohammed NI, et al. , Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: A population-based cohort study. Lancet Diabetes Endocrinol 2021; 9: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hydes T, Alam U, and Cuthbertson DJ, The impact of macronutrient intake on non-alcoholic fatty liver disease (nafld): Too much fat, too much carbohydrate, or just too many calories? Front Nutr 2021; 8: 640557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rinella ME and Sanyal AJ, Management of nafld: A stage-based approach. Nat Rev Gastroenterol Hepatol 2016; 13: 196–205. [DOI] [PubMed] [Google Scholar]


