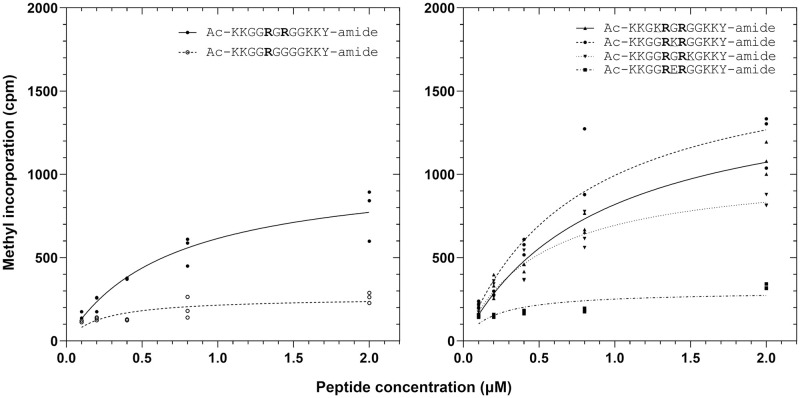
Fig 7. The effect of the RXR motif and charged residues on PRMT7-substrate enzyme kinetics.
The apparent binding affinity km and maximum initial activity Vmax of GST-HsPRMT7 with synthetic peptide substrates was modeled with Michaelis-Menten kinetics using data gathered by P81 phosphocellulose paper assay as described in Materials and Methods. Left, methylation of mono R compared to RGR substrates. Right, comparison of methylation of KRGR, RKR, RGRK, and RER substrates. 5 μg of GST-HsPRMT7 was incubated with various concentrations of the indicated peptide and 0.14 μM [3H]-AdoMet for 60 min at 25°C. All reactions indicated were run in triplicate. Human histone H2B (23–37) peptide was used as a positive control (not shown). Mono R, Ac-KKGGRGGGGKKY-amide; RGR, Ac-KKGGRGRGGKKY-amide; KRGR, Ac-KKGKRGRGGKKY-amide; RKR, Ac-KKGGRKRGGKKY-amide; RGRK, Ac-KKGGRGRKGKKY-amide; RER, Ac-KKGGRERGGKKY-amide.