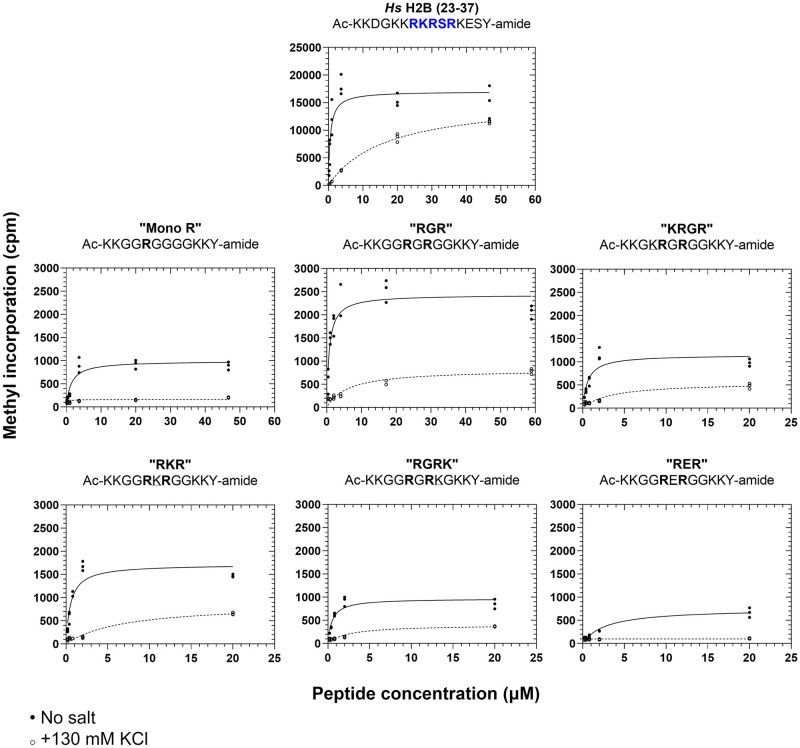
Fig 8. Increases in ionic strength affects PRMT7-substrate binding affinity and maximum initial reaction velocity.
The apparent binding affinity km and maximum initial activity Vmax of GST-HsPRMT7 with synthetic peptide substrates was modeled with Michaelis-Menten kinetics using data gathered by P81 phosphocellulose paper assay as described in Materials and Methods. For all reactions, 5 μg of GST-HsPRMT7 was incubated with various concentrations of the indicated peptide and 0.14 μM [3H]-AdoMet for 60 min at 25°C. Closed circle, methylation activity of human PRMT7 without added ionic strength. Open circle, methylation activity of human PRMT7 in the presence of 130 mM KCl. All reactions indicated were run in triplicate. Human histone H2B (23–37) peptide was used as a positive control for all experiments. Mono R, Ac-KKGGRGGGGKKY-amide; RGR, Ac-KKGGRGRGGKKY-amide; KRGR, Ac-KKGKRGRGGKKY-amide; RKR, Ac-KKGGRKRGGKKY-amide; RGRK, Ac-KKGGRGRKGKKY-amide; RER, Ac-KKGGRERGGKKY-amide.