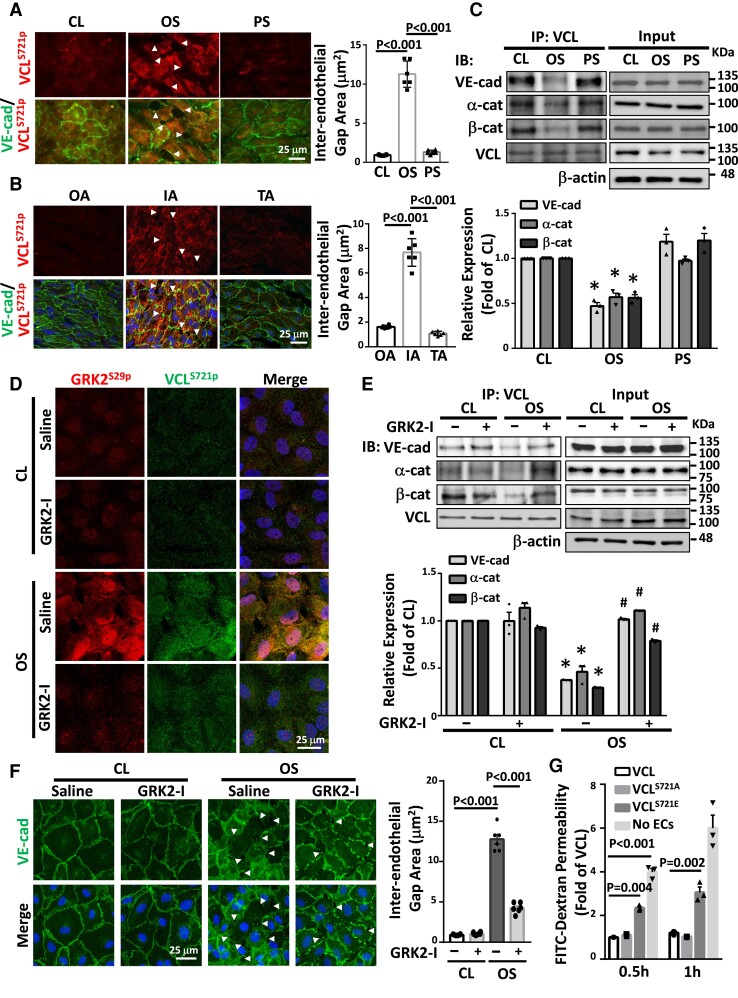
Figure 4.
Disturbed flow induction of VCLS721p increases EC permeability by disrupting VE-cadherin/catenin junctions. (A, C–F) Cultured ECs were kept under static condition as controls (CL) or subjected to oscillatory (OS) or pulsatile (PS) shear stress for 24 h. Cultured ECs (A) and EC monolayer in IA, OA, and TA of ApoE−/− mice (B) were en-face co-immunostained for VCLS721p and VE-cadherin. (C and E) EC lysates were immunoprecipitated with VCL-specific antibodies, followed by Western blot analysis for VCL, VE-cadherin, α-catenin, β-catenin. *P < 0.05 vs. CL. #P < 0.05 vs. saline OS. (D and F) Representative results of GRK2S29p and VCLS721p co-immunostaining (D) and VE-cadherin immunostaining (F) in ECs. Cell nuclei were counterstained with DAPI. In some experiments, ECs were pre-treated with a GRK2-specific inhibitor (GRK2-I, 30 μM) for 24 h (D–F). Arrowheads in A, B, and F indicate the gaps of inter-endothelial VE-cadherin junctions, and the gap areas were quantified. (G) Cultured ECs were transfected with VCLWT, VCLS721E, or VCLS721A, and their permeability was assessed using the in vitro endothelial permeability assay with FITC-conjugated dextran (70 kDa). n = 6 for each group in A, B, and F, and n = 3 for other results. Data are means ± SEM from three to six independent experiments. One-way ANOVA with the Tukey test was applied to A–C and G. Two-way ANOVA with the Tukey multiple comparison test was applied to E and F. Results represent three to six independent experiments with similar results.