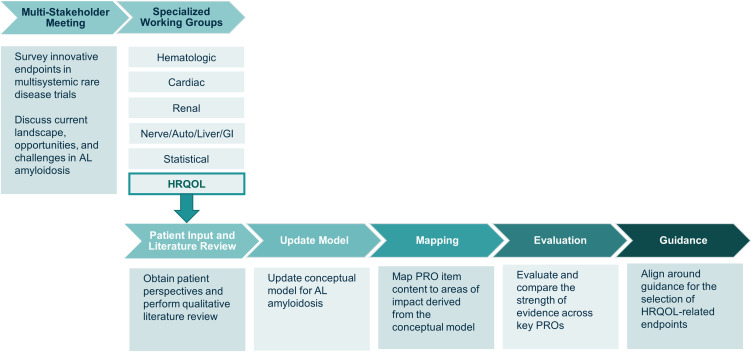
Figure 1.
The Amyloidosis Forum set out to develop a novel, multi-domain, composite endpoint and/or analysis methods for use in clinical trials for AL amyloidosis. Specialized working groups identified condition-specific and HRQOL outcome measures; an additional working group focused on statistical approaches to analysis of clinical trial data. The HRQOL Working Group sought to identify existing PRO instruments, deemed relevant across a broad spectrum of AL amyloidosis patients, for use in clinical trials and practice to facilitate patient-focused drug development in AL amyloidosis. Figure adapted from one presented at the Amyloidosis Forum meeting on January 22, 2021.22
Abbreviations: Auto, autonomic; GI, gastrointestinal; HRQOL, health-related quality of life; PRO, patient-reported outcome.