Abstract
Spinal cord injury (SCI), characterized by sensory disturbance and motor deficits, is associated with excessive inflammatory cytokine production of microglial cells. Previous studies have demonstrated High mobility group box 2 (HMGB2) as a microglial pro-inflammatory factor in stroke. This present study aims to evaluate the function of HMGB2 in a SCI rat model induced by striking the spinal cord at T9 to T12 using a rod. Our results showed that the levels of HMGB2 were significantly increased in the spinal cord tissues of SCI rats. Besides, HMGB2 downregulation was achieved by receiving an injection of lentivirus encoding HMGB2 shRNA in the spinal cord. Knockdown of HMGB2 suppressed SCI-induced microglial activation and neuroinflammation, as well as alleviated neuronal loss. In addition, we confirmed that HMGB2 silencing lessened lipopolysaccharide (LPS)-induced neuroinflammation in BV-2 cells. Furthermore, our findings demonstrated that HMGB2 knockdown suppressed the canonical nuclear factor of kB (NF-κB) signaling pathway both in vivo and in vitro. Collectively, this study manifested strong anti-inflammatory roles of HMGB2 knockdown on microglia-mediated neuroinflammation and suggested that HMGB2 might serve as a potential target for SCI therapy.
Keywords: High mobility group box 2, microglia, neuroinflammation, nuclear factor of κB, spinal cord injury
Introduction
Spinal cord injury (SCI) is defined as a devastating lesion to the spinal cord that temporarily or permanently leads to sensory, motor, and autonomic dysfunction [1]. The incidence of SCI is increasing globally with an annual approximated incidence of 10.4–83 per million people [2, 3]. As the biggest developing country with around 25% of the worldwide population, China has numerous SCI patients with an incidence of 14.6–60.6 per million [4]. Paralysis, chronic pain, and depression are the most common symptoms following SCI and are associated with severe physical, vocational, and social consequences for patients and families [5, 6].
Two injury processes, including primary injury and secondary injury, were involved in SCI. The primary injury consists of severed axons and broken micro-vessels as the initial physical damage, which triggers the secondary injury refers as a series of pathological events including neuronal apoptosis and neuroinflammation [7]. Among these, activation of microglia is closely connected with the process of SCI. Activated microglial cells induce multifarious inflammatory cytokines, which facilitate the influx of hematogenous macrophages and polymorphonuclear cells from the circulation, ultimately result in more serious damage of spinal cord [8]. Previous studies reported that elevated expression of IL-1β was observed in microglia and neurons after SCI, while IL-1 receptor antagonist treatment significantly decreased contusion-induced apoptosis in the spinal cord [9, 10]. Additionally, inhibition of TNF-α suppressed the inflammatory responses and tissue damages induced by spinal cord injury [11]. Therefore, treatments that restrained activation of microglia might represent a potential effective measure to alleviate tissue injury, consequently, improve the spinal cord function.
As a group of plentiful chromatin-binding proteins in the nucleus of mammal cells, High mobility group box proteins (HMGBs) contain a long acidic C-terminal domain and two DNA-binding HMGB domains, which bind distorted DNA [12, 13]. HMGBs are reported to function as universal sentinels, including extracellular signals secreted by both immune cells and damaged cells for nucleic acid-mediated innate immune responses [14, 15]. In particular, HMGB1 and HMGB2 proteins were the main research objects. Morteza demonstrated that the HMGB1/Nuclear factor of κB (NF-κB) signaling pathway was activated after SCI. Downregulation of the levels of HMGB1 and p-NF-κB suppressed the secondary phase SCI injuries in rats [16]. Meanwhile, because of its role in regulating gene expression, HMGB2 is associated with multiple biological processes, such as apoptosis and oxidative stress, especially inflammation [17, 18]. Noteworthiness, HMGB2 was identified as a microglial pro-inflammatory factor in stroke. Suppression of HMGB2 mitigated brain damage and improved neurological functions [19]. Additionally, a microglial inhibitor that targeted HMGB2 inhibited inflammation and reduced neuronal damage in an LPS-induced neuroinflammation mouse model [20]. Hence, HMGB2 might be associated with the occurrence and development of neuroinflammation. Nevertheless, little is known regarding the protective function of HMGB2 silencing in SCI.
This current study focused on the potential role of HMGB2 in microglia-mediated neuroinflammatory in SCI. We measured HMGB2 expression in the spinal cord tissues of SCI rats and investigated the pathological changes in SCI responses to HMGB2 knockdown. Furthermore, we also indicated the underlying mechanisms were associated with the anti-inflammatory roles of HMGB2 downregulation in BV-2 cells. Together, we hypothesize HMGB2 as the major microglial inflammatory factor and demonstrate the protective function of silencing-HMGB2 in SCI.
Materials and Methods
SCI and lentivirus injection
Female Wistar rats (8 weeks, 220–240 g) with free access to food and water were housed at a constant environmental temperature of 22°C with a 12:12-h light-dark cycle. All experimental protocols were approved by the Ethics Committee of Anhui Medical University. Rats were anesthetized with 2–3% isoflurane. For SCI models, the spine was exposed dorsally from T9 to T12 via an incision. Subsequently, a dorsal laminectomy was performed at the T10 level, followed by striking with a rod of 10 g and 3 mm in diameter on the exposed spine at a height of 25 mm. The impact caused a moderate T10 contusion injury. For sham controls, the spinal cord was exposed via dorsal laminectomy but the wound was immediately stitched closed. For lentiviral injections, a laminectomy was performed at T10, followed by receiving an intrathecal injection of HMGB2 shRNA (SCI+LV-shHMGB2) or negative control (SCI+LV-shNC) containing lentivirus (1 × 107 TU/ml, 8 µl, catalog number: BR004, FengHui Biotechnology, Changsha, China) by using a 30 G needle. The interference sequence of rat Hmgb2 was listed as follows: ccgGGAAGATGATGAAGATGAAGAttcaagagaTCTTCATCTTCATCATCTTCCttttt. Lentiviral delivery into the spinal cord was performed 3 days before SCI.
Behavioral assessment
The Basso Beattie Bresnahan (BBB) test from 0 (complete paralysis) to 21 (normal) was performed to assess locomotor functions of rats on days 3, 7, 14, and 28 postoperatively after surgery.
Morphometric study
The region of the lesion was fixed in 4% paraformaldehyde, followed placed in paraffin blocks and sectioned at a 5-µm thickness. Hematoxylin and eosin (H&E; hematoxylin, Solarbio Life Sciences, Beijing, China; eosin, Sangon Biotech, Shanghai, China) staining was used to access general histopathology of the spinal cord of rats. Luxol fast blue (LFB, Sigma-Aldrich, St. Louis, MO, USA) assay was performed to stain the myelinated axons. Cresyl violet (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) was used for staining of the Nissl substance in neurons. All the tissue sections were photographed using an Olympus microscope equipped with camera (Olympus Corp., Tokyo, Japan).
Cell culture
The mouse microglia cell line BV-2 cells were purchased from iCell Bioscience lnc (Shanghai, China) and maintained in DMEM medium (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) supplemented with 10% fetal bovine serum (Zhejing Tianhang Biotechnology Co., Ltd., Zhejiang, China). BV-2 cells were exposed to shHMGB2 (LPS+LV-shHMGB2) or negative control (LPS+LV-shNC) containing lentivirus for 16 h followed by the replacement of a fresh medium for incubation. The interference sequence of mouse Hmgb2 was listed as follows: ccgGCGAGGAGCACAAGAAGAAGCttcaagagaGCTTCTTCTTGTGCTCCTCGCttttt. After 3 days, cells were challenged with 1 µg/ml LPS for 24 h.
Immunofluorescent examination
Spinal cord sections and fixed BV-2 cells for immunofluorescence were incubated with the primary antibodies overnight at 4°C: Iba-1 (1:100, Abcam plc, Cambridge, MA, USA), HMGB2 (1:100, ABclonal Technology Co., Ltd., Wuhan, China), NeuN (1:100, Proteintech Group, lnc., Rosemont, IL, USA), and NF-κB p65 (1:100, ABclonal). After washing with PBS, the specimens were then incubated with the appropriate secondary antibodies: goat anti-rabbit IgG Alexa fluor 555 (1:200, Invitrogen, Carlsbad, CA, USA), goat anti-mouse IgG Alexa fluor 555 (1:200, Invitrogen), and goat anti-rabbit IgG FITC (1:200, Abcam plc). The slices were counterstained with 4’, 6-Diamidine-2’-phenylindole dihydrochloride (DAPI) and captured by a fluorescent microscope (Olympus).
Real-time PCR analysis
TRIpure reagent (BioTeke Corp., Beijing, China) was used to isolate the total RNA from injured spinal cord tissues and BV-2 cells. cDNA was synthesized using BeyoRT II M-MLV (Beyotime Biotechnology, Shanghai, China) after Rnase inhibitor treatment (BioTeke Corp.) and amplified using SYBR Green (Solarbio Life Sciences) and real-time system (Bioneer Corp., Daejeon, Korea) according to the manufacturer’s instructions. Primers were listed as follows: rat Hmgb2 forward, 5′-CTCGTCGGTCAACTTCGC-3′, reverse, 5′-TGGGAGCATTTGGATCTTTT-3′; mouse IL-1 β forward, 5′-CTCAACTGTGAAATGCCACC-3′, reverse, 5′-GAGTGATACTGCCTGCCTGA-3′; mouse IL-6 forward, 5′- ATGGCAATTCTGATTGTATG-3′, reverse, 5′- GACTCTGGCTTTGTCTTTCT-3′; mouse TNF-α forward, 5′- CAGGCGGTGCCTATGTCTCA-3′, reverse, 5′- GCTCCTCCACTTGGTGGTTT-3′; rat β-actin forward, 5′- GGAGATTACTGCCCTGGCTCCTAGC-3′, reverse, 5′- GGCCGGACTCATCGTACTCCTGCTT-3′; mouse β-actin forward, 5′- CTGTGCCCATCTACGAGGGCTAT-3′, reverse, 5′- TTTGATGTCACGCACGATTTCC-3′. Relative mRNA transcript expressions for target genes were determined using the ΔΔCT method, relative to the reference gene, β-actin.
Western blot analysis
Homogenates of injured spinal cord tissues and lysates of BV-2 cells were prepared using a lysis buffer (Beyotime Biotechnology) supplemented with 1 mmol/L phenylmethanesulfonyl fluoride (PMSF, Beyotime Biotechnology). Equal amounts of 40 µg protein were loaded onto 14% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with 5% skim milk, the membranes were incubated with primary antibodies: HMGB2 (1:2,000, ABclonal Technology Co., Ltd.), p-NF-κB p65 (Ser536, 1:500, Cell Signaling Technology, Danvers, MA, USA), NF-κB p65 (1:1,000, ABclonal Technology Co., Ltd.), p-IκBα (1:1,000, Cell Signaling Technology), and IκBα (1:1,000, Cell Signaling Technology). The membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000, Beyotime Biotechnology). The blotted protein bands were detected by using an enhanced chemiluminescence solution (ECL, Beyotime Biotechnology). Histone H3 and β-actin were used as the normalization controls.
ELISA
The levels of TNF-α, IL-1β, and IL-6 in injured spinal cord tissues and BV-2 cells were measured using commercial ELISA kits (Multi Sciences (Lianke) Biotech, Co., Ltd., Hangzhou, China).
Statistics
Statistical analysis was carried out by GraphPad Prism 8.0. The results were expressed as means ± SD. For data comparison, one-way and two-way analyses of variance (ANOVA) followed by Tukey’s or Bonferroni’s post hoc test were used. A level of P<0.05 was considered significant.
Results
HMGB2 expression was elevated in the spinal cord tissues of rats following SCI
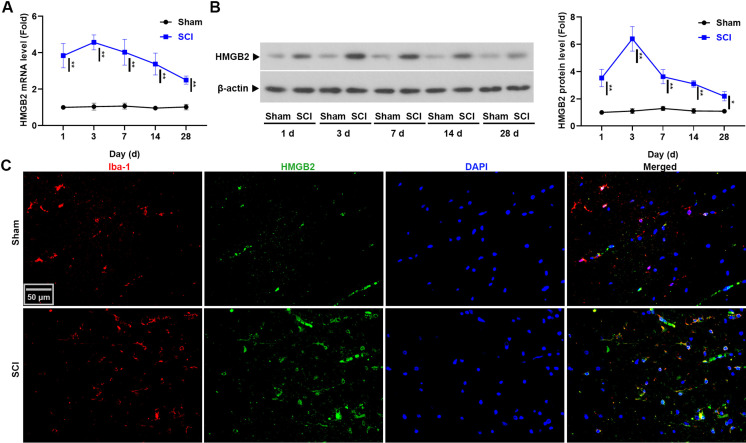
The time course of changes in the levels of HMGB2 in injured spinal cord tissues were firstly shown in Figs. 1A and B. Compared with the sham rats, the mRNA and protein levels of HMGB2 in the spinal cord tissues were significantly increased in SCI rats, especially at day 3: 4.57 ± 0.41-fold vs 1.04 ± 0.19-fold (P<0.01) and 6.40 ± 0.90-fold vs 1.10 ± 0.19-fold (P<0.05), respectively. Furthermore, the results of real-time PCR and western blot were confirmed by immunofluorescence (Fig. 1C). High level of HMGB2 colocalization with the microglial cell marker Iba-1 was observed in the spinal cord tissues of rats with SCI (n=3). These findings demonstrated that SCI induced sustained upregulation of HMGB2 in spinal cord tissues.
Fig. 1.
High mobility group box 2 (HMGB2) was upregulated in the spinal cord tissues of rats. (A) The mRNA level of Hmgb2 was detected using real-time PCR. (B) Western blot was used to test the protein level of HMGB2. Data are presented as the mean ± SD (n=3) and were analyzed using two-way analyses of variance (ANOVA) followed by Bonferroni’s post hoc test, *P<0.05, **P<0.01. (C) Co-labeling of Iba-1 with HMGB2 in the spinal cord tissues of spinal cord injury (SCI) rats.
HMGB2 silencing improved neuronal survival in rats with SCI
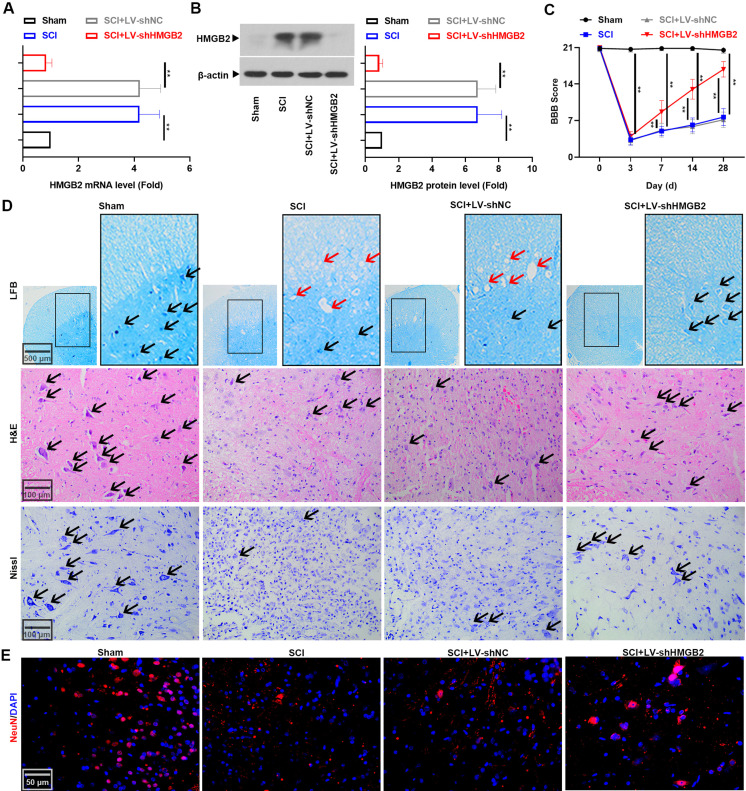
HMGB2 deficiency was reported to promote the proliferation of embryonic neural stem cells (NSC) [21]. Therefore, we postulated that silenced HMGB2 expression in the spinal cord might facilitate neural regeneration after SCI. Lentivirus-mediated HMGB2 downregulation in the spinal cord tissues at 3 days post-injury was confirmed by real-time PCR and western blot (Figs. 2A and B). Rod striking on the exposed spine resulted in hindlimb paralysis, with BBB scores at day 3 after injury of 3.33 ± 1.03 in the SCI group. Gradual recovery in hindlimb function in the SCI+LV-shHMGB2 group as reflected in the higher scores were observed on subsequent test days (Fig. 2C, P<0.01). Figure 2D showed the anatomical changes within all the experimental groups. Sham rats did not exhibit any tissue disruption of the spinal cord. However, rats with SCI presented decreased neurons (black arrows) and increased demyelination vacuolations (red arrows). In contrast, lentivirus encoding shHMGB2 treatment promoted neuronal survival and axonal regeneration. The pathological results were confirmed by immunostaining, which showed that NeuN (a neuron-specific nuclear protein) expression in the spinal cord tissues was elevated in LV-shHMGB2 treated rats with SCI (Fig. 2E) (n=6). Collectively, these results showed the protection of the spinal cord tissue and the improvement in motor function were associated with HMGB2 knockdown.
Fig. 2.
High mobility group box 2 (HMGB2) knockdown alleviated neuronal loss 3 days after spinal cord injury (SCI). (A) Real-time PCR was performed to determine the mRNA level of Hmgb2. (B) The protein level of HMGB2 was accessed using Western blot. Data are expressed as means ± SD (n=6) and were compared among groups using one-way analyses of variance (ANOVA) followed by Tukey’s post hoc test, **P<0.01. (C) The Basso Beattie Bresnahan (BBB) score of rats with SCI. Two-way ANOVA followed by Tukey’s post hoc test. Mean ± SD (n=6) **P<0.01. (D) LFB, H&E, and Nissl staining of the spinal cord tissues of rats with SCI. Black arrows showed neurons and red arrows showed demyelination vacuolations. (E) Specificity of NeuN was detected in the spinal cord tissues.
Downregulation of HMGB2 inhibited SCI-induced neuroinflammation
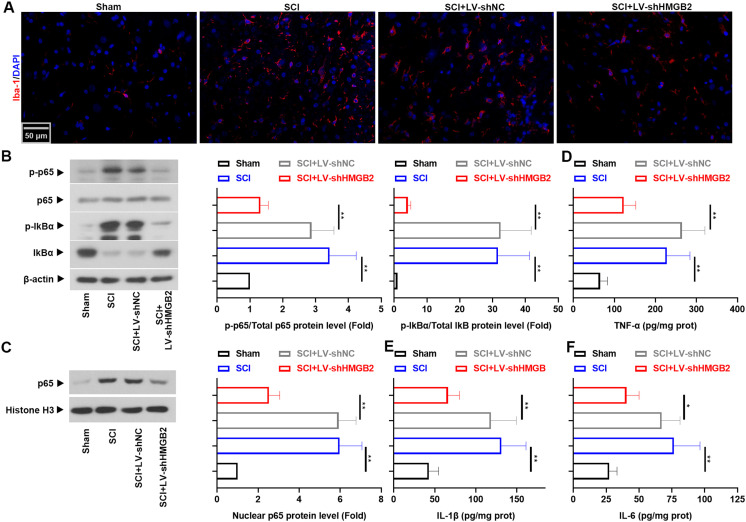
Given the role of HMGB2 in microglia-mediated neuroinflammation [20], microglial activation in the injured spinal cord was monitored, based on changes in the microglial cell marker Iba-1, the transcription factor NF-κB p65, and the inflammatory factors TNF-α, IL-1β, and IL-6. We noted an increase in the levels of Iba-1 that paralleled the phosphorylation of NF-κB p65 and the upregulation of inflammatory cytokines. However, these changes were reversed by HMGB2 knockdown (Fig. 3, P<0.05) (n=6). Together, these data suggested that HMGB2 silencing played an anti-neuroinflammatory role in the recovery of SCI.
Fig. 3.
Reduction of High mobility group box 2 (HMGB2) restrained neuroinflammation. Immunofluorescence images of Iba-1 in the spinal cord tissues of spinal cord injury (SCI) rats. (B) The protein levels of p-NF-κB p65, NF-κB p65, p-IκBα, IκBα, and (C) nuclear NF-κB p65 were analyzed by Western blot analysis. Concentrations of (D) TNF-α, (E) IL-1β, and (F) IL-6 were evaluated using ELISA. All results are presented as the mean ± SD (n=6) and assessed by one-way analyses of variance (ANOVA) followed by Tukey’s post hoc test, *P<0.05, **P<0.01.
HMGB2 knockdown suppressed microglial activation and expression of inflammatory proteins in BV-2 cells stimulated with LPS
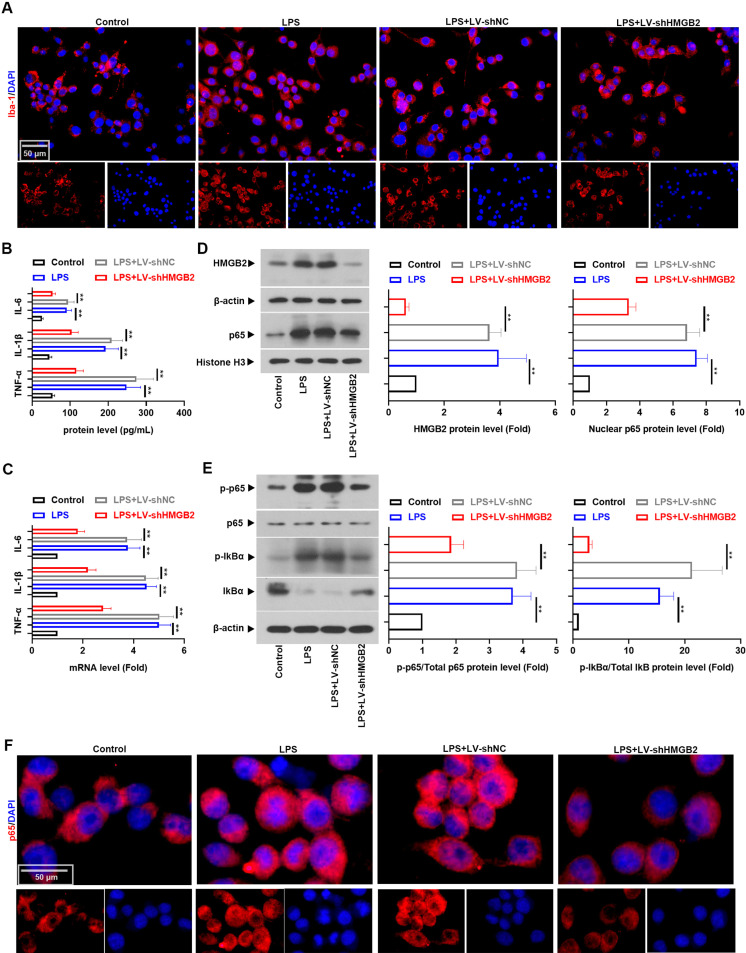
To access the function of HMGB2 on microglial activation in vitro, we selected BV-2 cells in the following experiments. Data in Figs. 4A–C depicted that LPS treatment upregulated the levels of TNF-α, IL-1β, and IL-6 as well as the expression of Iba-1 (P<0.01). Similarly, LPS promoted the activation of NF-κB p65 in BV-2 cells (Figs. 4D–F, P<0.01). As expected, downregulation of HMGB2 could impede these alterations (n=3). These results indicated that HMGB2 knockdown counteracted LPS-induced microglial activation and inflammation.
Fig. 4.
High mobility group box 2 (HMGB2) silencing decreased lipopolysaccharide (LPS)-induced inflammation in BV-2 cells. (A) Immunofluorescence staining of Iba-1 in BV-2 cells. (B) The levels of TNF-α, IL-1β, and IL-6 in supernatants collected from BV-2 cells were evaluated using ELISA. (C) Real-time PCR was used to test the mRNA levels of TNF-α, IL-1β, and IL-6. Western blot analysis was used to monitor the protein expression of (D) HMGB2 and nuclear NF-κB p65, (E) p-NF-κB p65, NF-κB p65, p-IκBα, and IκBα. (F) Immunofluorescence staining of NF-κB p65 in BV-2 cells. All number values are reported as the mean ± SD (n=3) and all data were analyzed using one-way analyses of variance (ANOVA) followed by Tukey’s post hoc test, **P<0.01.
Discussion
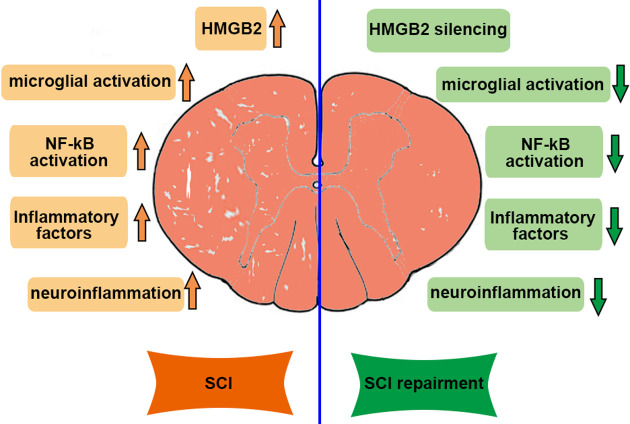
The principal findings of this present work were the protective role of downregulated-HMGB2 in SCI. Increased expression of HMGB2 was observed in the spinal cord tissues of SCI rats and the elevation was accompanied by the activation of microglial cells. Moreover, HMGB2 knockdown mitigated histopathological lesions and improved neurologic outcomes. Further, we showed that HMGB2 silencing inhibited neuroinflammation, which is connected with the inactivation of the NF-κB signaling pathway (Fig. 5). Understanding the function of HMGB2 in SCI informs theoretical bases for using silenced-HMGB2 to accelerate neural restoration after injury.
Fig. 5.
The function of High mobility group box 2 (HMGB2) in spinal cord injury (SCI). Knockdown of HMGB2 attenuated SCI by suppressing microglia activation and neuroinflammation via inhibiting the NF-κB signaling pathway.
The HMGB family is composed of four members: HMGB1, 2, 3, and 4. Both HMGB1 and HMGB2 exhibited ubiquitous and expressed at high levels during mouse embryonic development [22, 23]. Nevertheless, expressions of HMGB3 and HMGB4 were more localized during embryogenesis [24, 25]. Interestingly, HMGB1, 2, 3, and 4 were all expressed in proliferating NSC at E12.5 of mouse embryos, which indicated possible roles for these proteins in NSC proliferation in embryonic mice and neural restoration in the adult following injury. Notably, a decrease in HMGB2 mRNA expression in proliferating NSC between E12 and E15.5 was observed, so it was possible that HMGB2 played a negative regulatory role during neural development [21]. In agreement with the above results, an important finding of our data is the elevation in HMGB2 level in injured spinal cord tissues of SCI rats. HMGB2 deficiency facilitated neuronal survival and motor function. These positive results supported that HMGB2 might be a potential target for SCI therapy. Besides, strong biochemical and morphological evidences showed the occurrence of apoptosis in neurons following SCI [26]. A previous study reported that knockdown of HMGB2 suppressed cell apoptosis and facilitated the expression of Bcl-2, identified as an anti-apoptotic factor [27]. Therefore, HMGB2 silencing might improve neural survival and functional outcome via inhibiting apoptosis after SCI.
Microglia are resident immune cells of the central nervous system, which are responsible for the safeguard of neural cells from insults, as well as for the homeostasis of the spinal cord tissue [28]. In the case of SCI, the initial insult facilitates the proliferation and activation of microglial cells and the induction of pro-inflammatory cytokines, subsequently resulting in neuroinflammation. A recent study indicated that suppressing microglia-mediated neuroinflammation was a potential therapeutic strategy for neuropathic pain in the spinal cord of mice [29]. Diminishing the robust neuroinflammatory response and the detrimental immune signaling could reduce tissue damage and accelerate functional recovery following SCI [30]. In line with these findings, HMGB2 knockdown inhibited SCI-induced microglial activation. It is well known that the pro-inflammatory phenotype (M1) and anti-inflammatory phenotype (M2) maintain spinal cord homeostasis [31]. During the acute phase of neuroinflammation, the blood-spinal cord barrier at the site of the SCI is infiltrated by M1 microglia, which secrete multiple inflammatory cytokines, such as TNF-α, IL-1 β, and IL-6 [32]. The microglia phenotype changes to M2 following the activation of M1 microglia contributes to anti-inflammatory and neuroprotective effects [33]. As expected, the present results showed that HMGB2 downregulation reduced the levels of TNF-α, IL-1 β, and IL-6 in the spinal cord tissues of SCI rats. Hence, HMGB2 might exert a crucial role in regulating microglia polarization after SCI. Furthermore, suppressing microglia proliferation after SCI accelerates recovery in mice [34]. HMGB2 silencing was observed to inhibit the proliferation of microvascular endothelial cells and hepatocytes [12, 35]. Further studies are needed to confirm whether HMGB2 reduces the release of inflammatory cytokines by suppressing the proliferative capacity of microglia. In addition, microglia switch from the M1 state to the M2 state under HMGB2 silencing also need thorough investigation.
In the central nervous system, the canonical NF-κB (p65) pathway is particularly connected with the production of chemokines and inflammatory cytokines. The NF-κB signaling pathway is one of the vital pathways in the infiltration of microglia into the injury site during activated immune responses after SCI. Under normal conditions, the transcription factor NF-κB is in the inactive state and principally located in the cytosol, due to the formation of the complex with the inhibitory subunit (IκB). After specific stimulation, including biological factors such as TNF-α and IL-1 β, NF-κB is released from the cytoplasm to the nucleus by phosphorylated and degraded IκB, subsequently modulating the levels of NF-κB-dependent inflammatory factors [36, 37]. NF-κB translocation in the nuclear fractions in the spinal cord tissues of SCI mice was reported [38]. HMGBs were reported to be involved in the activation of NF-κB. HMGB1 could promote glial phagocytic activity that in turn activated NF-κB with subsequent inflammatory cytokine production [39]. Notably, our findings indicated that HMGB2 knockdown inhibited SCI-induced activation of the NF-κB signaling pathway. This is in line with the previous study showing that HMGB2 downregulation inhibited inflammation and the NF-κB signaling pathway [40]. HMGB2 facilitated brain injury via activating the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway [41]. In light of this finding, it is reasonable to assume that elevated HMGB2 level was associated with a remarkable increase in the activation of NF-κB, subsequently inducing neuroinflammation in SCI. Furthermore, as the priority drug used in SCI, methylprednisolone exerted its anti-inflammatory property, which suppressed TNF-α expression and NF-κB activation in SCI rats [42,43,44]. This present study showed that silencing HMGB2 had an anti-inflammatory function, which might provide theoretical bases in developing the treatment of SCI. HMGB1 was reported to partially complement the absence of HMGB2 [45]. However, in the present study, the decreases in p-NF-κB p65 induced by LPS were almost completely abolished by siRNA-mediated knockdown of HMGB2, which might be associated with the specifical roles of HMGB1 and HMGB2 in SCI. To address these limitations, the interaction between HMGB1 and HMGB2 after SCI remains to be elucidated.
In summary, this study demonstrated for the first time that knockdown of HMGB2 supported the beneficial effects of motor functional recovery following SCI in rats. The protective roles of HMGB2 silencing might be associated with the suppression of microglia-mediated neuroinflammation and the inactivation of the NF-κB signaling pathway.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
None.
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017; 3: 17018. doi: 10.1038/nrdp.2017.18 [DOI] [PubMed] [Google Scholar]
- 2.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015; 24: 905–918. doi: 10.1007/s00586-014-3424-6 [DOI] [PubMed] [Google Scholar]
- 3.Yuan S, Shi Z, Cao F, Li J, Feng S. Epidemiological Features of Spinal Cord Injury in China: A Systematic Review. Front Neurol. 2018; 9: 683. doi: 10.3389/fneur.2018.00683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Qiao X, Liu W, Fekete C, Reinhardt JD. Epidemiology of spinal cord injury in China: A systematic review of the chinese and english literature. Spinal Cord. 2022; 60: 1050–1061. doi: 10.1038/s41393-022-00826-6 [DOI] [PubMed] [Google Scholar]
- 5.Hunter Revell SM. Symptom clusters in traumatic spinal cord injury: an exploratory literature review. J Neurosci Nurs. 2011; 43: 85–93. doi: 10.1097/JNN.0b013e31820c2533 [DOI] [PubMed] [Google Scholar]
- 6.Yahanda AT, Chicoine MR. Paralysis Caused by Spinal Cord Injury After Posterior Fossa Surgery: A Systematic Review. World Neurosurg. 2020; 139: 151–157. doi: 10.1016/j.wneu.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Deumens R, Koopmans GC, Joosten EA. Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol. 2005; 77: 57–89. doi: 10.1016/j.pneurobio.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 8.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011; 12: 388–399. doi: 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 2004; 29: 966–971. doi: 10.1097/00007632-200405010-00004 [DOI] [PubMed] [Google Scholar]
- 10.Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001; 18: 947–956. doi: 10.1089/089771501750451857 [DOI] [PubMed] [Google Scholar]
- 11.Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E, et al. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008; 29: 32–41. doi: 10.1097/shk.0b013e318059053a [DOI] [PubMed] [Google Scholar]
- 12.Jo HR, Jeong JH. MicroRNA-Mediated Downregulation of HMGB2 Contributes to Cellular Senescence in Microvascular Endothelial Cells. Cells. 2022; 11: 584. doi: 10.3390/cells11030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001; 29: 395–401. doi: 10.1042/bst0290395 [DOI] [PubMed] [Google Scholar]
- 14.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009; 462: 99–103. doi: 10.1038/nature08512 [DOI] [PubMed] [Google Scholar]
- 15.Block ML. Neuroinflammation: modulating mighty microglia. Nat Chem Biol. 2014; 10: 988–989. doi: 10.1038/nchembio.1691 [DOI] [PubMed] [Google Scholar]
- 16.Gholaminejhad M, Jameie SB, Abdi M, Abolhassani F, Mohammed I, Hassanzadeh G. All-Trans Retinoic Acid-Preconditioned Mesenchymal Stem Cells Improve Motor Function and Alleviate Tissue Damage After Spinal Cord Injury by Inhibition of HMGB1/NF-κB/NLRP3 Pathway Through Autophagy Activation. J Mol Neurosci. 2022; 72: 947–962. doi: 10.1007/s12031-022-01977-0 [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Zhang H, Zhang Q, Xu J. β-Amyrin ameliorates diabetic nephropathy in mice and regulates the miR-181b-5p/HMGB2 axis in high glucose-stimulated HK-2 cells. Environ Toxicol. 2022; 37: 637–649. doi: 10.1002/tox.23431 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Liu X, Jiang Y, Wang N, Li F, Xin H. 6-Gingerol Attenuates Ischemia-Reperfusion-Induced Cell Apoptosis in Human AC16 Cardiomyocytes through HMGB2-JNK1/2-NF-κB Pathway. Evid Based Complement Alternat Med. 2019; 2019: 8798653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Wu Z, He A, Zhang T, Zhang P, Jin J, et al. Genome-Wide Screen and Validation of Microglia Pro-Inflammatory Mediators in Stroke. Aging Dis. 2021; 12: 786–800. doi: 10.14336/AD.2020.0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Nam Y, Koo JY, Lim D, Park J, Ock J, et al. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol. 2014; 10: 1055–1060. doi: 10.1038/nchembio.1669 [DOI] [PubMed] [Google Scholar]
- 21.Abraham AB, Bronstein R, Chen EI, Koller A, Ronfani L, Maletic-Savatic M, et al. Members of the high mobility group B protein family are dynamically expressed in embryonic neural stem cells. Proteome Sci. 2013; 11: 18. doi: 10.1186/1477-5956-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauken CM, Nagle DL, Bucan M, Lo CW. Molecular cloning, expression analysis, and chromosomal localization of mouse Hmg1-containing sequences. Mamm Genome. 1994; 5: 91–99. doi: 10.1007/BF00292334 [DOI] [PubMed] [Google Scholar]
- 23.Ronfani L, Ferraguti M, Croci L, Ovitt CE, Schöler HR, Consalez GG, et al. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development. 2001; 128: 1265–1273. doi: 10.1242/dev.128.8.1265 [DOI] [PubMed] [Google Scholar]
- 24.Moleri S, Cappellano G, Gaudenzi G, Cermenati S, Cotelli F, Horner DS, et al. The HMGB protein gene family in zebrafish: Evolution and embryonic expression patterns. Gene Expr Patterns. 2011; 11: 3–11. doi: 10.1016/j.gep.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 25.Vaccari T, Beltrame M, Ferrari S, Bianchi ME. Hmg4, a new member of the Hmg1/2 gene family. Genomics. 1998; 49: 247–252. doi: 10.1006/geno.1998.5214 [DOI] [PubMed] [Google Scholar]
- 26.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000; 17: 915–925. doi: 10.1089/neu.2000.17.915 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Zhang Y, Ding K, Zhang H, Zhao Q, Liu Z, et al. Involvement of JNK1/2-NF-κBp65 in the regulation of HMGB2 in myocardial ischemia/reperfusion-induced apoptosis in human AC16 cardiomyocytes. Biomed Pharmacother. 2018; 106: 1063–1071. doi: 10.1016/j.biopha.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 28.Kroner A, Rosas Almanza J. Role of microglia in spinal cord injury. Neurosci Lett. 2019; 709: 134370. doi: 10.1016/j.neulet.2019.134370 [DOI] [PubMed] [Google Scholar]
- 29.Koshimizu H, Ohkawara B, Nakashima H, Ota K, Kanbara S, Inoue T, et al. Zonisamide ameliorates neuropathic pain partly by suppressing microglial activation in the spinal cord in a mouse model. Life Sci. 2020; 263: 118577. doi: 10.1016/j.lfs.2020.118577 [DOI] [PubMed] [Google Scholar]
- 30.Lund MC, Ellman DG, Nissen M, Nielsen PS, Nielsen PV, Jørgensen C, et al. The Inflammatory Response after Moderate Contusion Spinal Cord Injury: A Time Study. Biology (Basel). 2022; 11: 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhmetzyanova E, Kletenkov K, Mukhamedshina Y, Rizvanov A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front Syst Neurosci. 2019; 13: 37. doi: 10.3389/fnsys.2019.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asano K, Nakamura T, Funakoshi K. Early mobilization in spinal cord injury promotes changes in microglial dynamics and recovery of motor function. IBRO Neurosci Rep. 2022; 12: 366–376. doi: 10.1016/j.ibneur.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014; 9: 1787–1795. doi: 10.4103/1673-5374.143423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulen G, Aloy E, Bringuier CM, Mestre-Francés N, Artus EVF, Cardoso M, et al. Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics. 2021; 11: 8640–8659. doi: 10.7150/thno.61833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yano K, Choijookhuu N, Ikenoue M, Fidya, Fukaya T, Sato K, et al. Spatiotemporal expression of HMGB2 regulates cell proliferation and hepatocyte size during liver regeneration. Sci Rep. 2022; 12: 11962. doi: 10.1038/s41598-022-16258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mincheva-Tasheva S, Soler RM. NF-κB signaling pathways: role in nervous system physiology and pathology. Neuroscientist. 2013; 19: 175–194. doi: 10.1177/1073858412444007 [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002; 2: 725–734. doi: 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- 38.Lanza M, Campolo M, Casili G, Filippone A, Paterniti I, Cuzzocrea S, et al. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol Neurobiol. 2019; 56: 3937–3947. doi: 10.1007/s12035-018-1347-7 [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Hu L, Jiang J, Li H, Wu Q, Ooi K, et al. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflammation. 2020; 17: 15. doi: 10.1186/s12974-019-1673-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Zhao Z, Zhao X, Xie H, Zhang C, Sun X, et al. HMGB2 causes photoreceptor death via down-regulating Nrf2/HO-1 and up-regulating NF-κB/NLRP3 signaling pathways in light-induced retinal degeneration model. Free Radic Biol Med. 2022; 181: 14–28. doi: 10.1016/j.freeradbiomed.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Hong Z, Zhang L, Guo J, Li Y, Li K. HMGA2 Promotes Brain Injury in Rats with Cerebral Infarction by Activating TLR4/NF-κB Signaling Pathway. Mediators Inflamm. 2022; 2022: 1376959. doi: 10.1155/2022/1376959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JZ, Yang M, Meng M, Li ZH. Clinical characteristics and treatment of spinal cord injury in children and adolescents. Chin J Traumatol. 2023; 26: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997; 277: 1597–1604. doi: 10.1001/jama.1997.03540440031029 [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998; 59: 135–142. doi: 10.1016/S0169-328X(98)00142-9 [DOI] [PubMed] [Google Scholar]
- 45.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999; 19: 5237–5246. doi: 10.1128/MCB.19.8.5237 [DOI] [PMC free article] [PubMed] [Google Scholar]