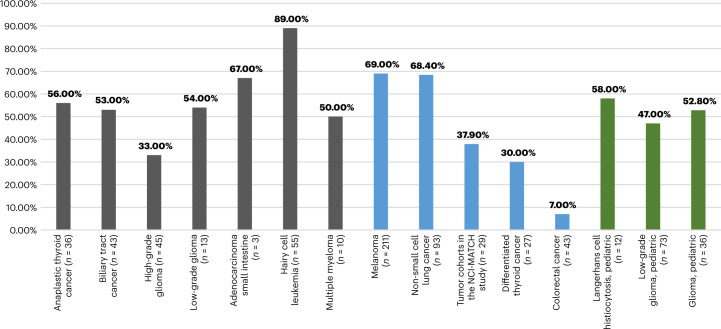
Fig. 2. ORR with dabrafenib plus trametinib in cancers with BRAFV600E mutations.
This figure presents data for the ROAR study (gray) and other studies in adults (blue) and the pediatric population (green). Data are presented for the COMBI-d study (NCT01584648) for melanoma, BRF113928 (NCT01336634) for NSCLC, NCT01723202 for differentiated thyroid cancer and ROAR (NCT02034110) and NCI-MATCH (NCT02465060) for the other tumor types. Pediatric data for gliomas and Langerhans cell histiocytosis are from the study NCT02124772, and those for LGGs are from the study NCT02684058. Data for colorectal cancer are from the study NCT01072175 (data on file, Novartis). The NCT01584648 study included previously untreated patients; NCT01723202 included patients who were refractory to radioactive iodine; NCT01336634 included both previously treated and treatment-naive patients; and other studies included patients who previously received standard treatment. Patient numbers are the patients who received treatment with dabrafenib plus trametinib. The ORR for GIST (n = 1) in the ROAR study was 0. Patients with melanoma, thyroid cancer, colorectal cancer and NSCLC were excluded in the NCI-MATCH study. This study included patients with BRAFV600E-positive tumors of the gastrointestinal tract (n = 11), lung (n = 5), CNS (n = 5), myeloma (n = 1), ameloblastoma of the mandible (n = 1) and gynecologic malignancies (n = 6). In the NCI-MATCH study, no CRs were observed; durable PRs were seen across a variety of tumor types, including papillary adenocarcinoma of the lung (n = 5), low-grade serous ovarian carcinoma (n = 5), mucinous-papillary serous adenocarcinoma of the peritoneum (n = 1), histiocytic sarcoma of the brain (n = 1), pleomorphic xanthoastrocytoma of the parietal lobe (n = 1) and cholangiocarcinoma (n = 4).