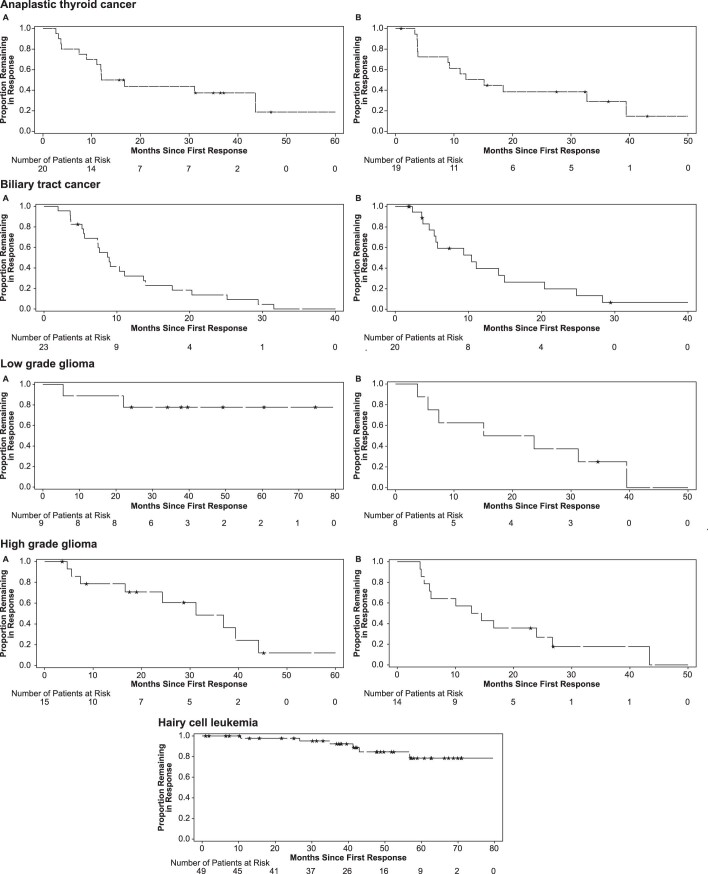
Extended Data Fig. 2. Duration of response by investigator and independent radiology assessment across tumor cohorts.
Efficacy evaluable population; Primary and expansion cohorts; (A) Investigator assessment (B) Independent radiology assessment. ATC: Among 20 responders by investigator assessment, nine (45%) patients had disease progression and four (20%) died. Seven (35%) patients ended their follow-up and were censored. Among 19 responders by independent radiology assessment, 12 (63%) patients had disease progression and one (5%) died. Six (32%) patients ended their follow-up and were censored. BTC: Among 23 responders by investigator assessment, 20 (87%) patients had disease progression and 2 (9%) died. One (4%) patient was censored due to end of follow-up. Among the 20 responders by independent radiology assessment, 14 (70%) patients had disease progression and one (5%) died. Five (25%) patients were censored due to end of follow-up. LGG: Among nine responders by investigator assessment, two (22%) patients had disease progression. Seven (78%) patients were censored due to end of follow-up. Among eight responders by independent radiology assessment, seven (88%) patients had disease progression. One (13%) patient was censored due to end of follow-up. HGG: Among 15 responders by investigator assessment, nine (60%) patients had disease progression. Six (40%) patients were censored due to end of follow-up. Among 14 responders by independent radiology assessment, 12 (86%) patients had disease progression. Two (14%) patients were censored due to end of follow-up. ASI: Due to small numbers of responders, KM curves are not shown. Of the two responders by investigator assessment, one patient had disease progression and one was censored due to end of follow-up. Among the two responders by independent radiology assessment, both patients had disease progression. HCL: Among 49 responders by investigator assessment, four (8%) patients had disease progression and two (4%) patients died. Forty-three patients (88%) were censored due to end of follow-up. MM: Due to small numbers of responders, KM curves are not shown. Among five responders by investigator assessment, 4 (80%) patients had disease progression. One (20%) patient was censored due to end of follow-up. ASI, adenocarcinoma of small intestine; ATC, anaplastic thyroid cancer; BTC, biliary tract cancer; G, grade; HCL, hairy cell leukemia; HGG, high (WHO G3/G4) grade glioma; KM, Kaplan-Meier; LGG, low (WHO G1/G2) grade glioma; MM, multiple myeloma; WHO, World Health Organization.