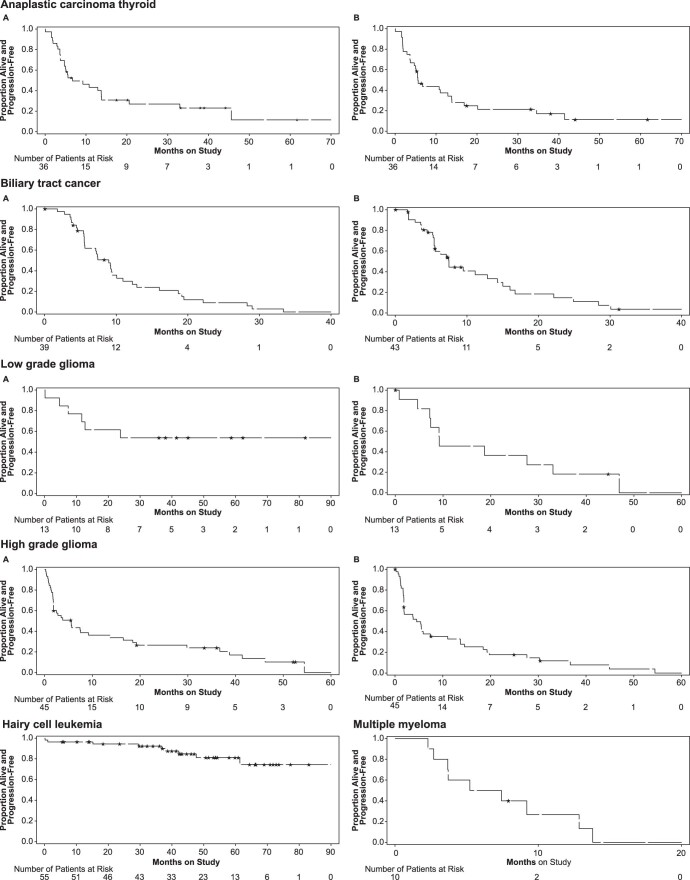
Extended Data Fig. 3. Progression free survival by investigator and independent radiology assessment across tumor cohorts.
Efficacy evaluable population: Primary and expansion cohorts in all except low grade glioma and MM which is primary analysis cohort; (A) Investigator assessment (B) Independent radiology assessment. ATC: By investigator assessment in 36 patients, 21 (58%) patients had disease progression and six (17%) had died without disease progression. Nine (25%) patients were censored due to end of follow-up. By independent radiology review, progression of disease was observed in 26 (72%) patients and three (8%) patients died without disease progression. Seven (19%) patients were censored due to end of follow-up. BTC: By investigator assessment in 43 patients, 33 (77%) patients had disease progression and six (14%) patients had died without disease progression. Four patients were censored due to end of follow-up. By independent radiology review, progression of disease was observed in 27 (63%) patients and five (12%) patients died without disease progression. Eleven (26%) patients were censored due to end of follow-up. ASI: By investigator assessment in three patients, one patient (33%) had disease progression. Two (67%) patients were censored due to end of follow-up. By independent radiology review, progression of disease was observed in three (100%) patients. LGG: By investigator assessment in 13 patients, six (46%) patients had disease progression. Seven (54%) patients were censored due to end of follow-up. By independent radiology review, progression of disease was observed in nine (69%) patients and one (8%) patient died without disease progression. Three (23%) patients were censored due to end of follow-up. HGG: By investigator assessment in 45 patients, 38 (84%) patients had disease progression. Seven (16%) patients were censored due to end of follow-up. By independent radiology review, progression of disease was observed in 36 (80%) patients and four (9%) patients died without disease progression. Five (11%) patients were censored due to end of follow-up. HCL: By investigator assessment in 55 subjects, six (11%) patients had disease progression and three (5%) patients had died without disease progression. Forty-six (84%) patients were censored due to end of follow-up. MM: By investigator assessment in 10 patients, nine (90%) patients had disease progression. One patient was censored due to end of follow-up. ASI, adenocarcinoma of small intestine; ATC, anaplastic thyroid cancer; BTC, biliary tract cancer; G, grade; HCL, hairy cell leukemia; HGG, high (WHO G3/G4) grade glioma; LGG, low (WHO G1/G2) grade glioma; MM, multiple myeloma; WHO, World Health Organization.