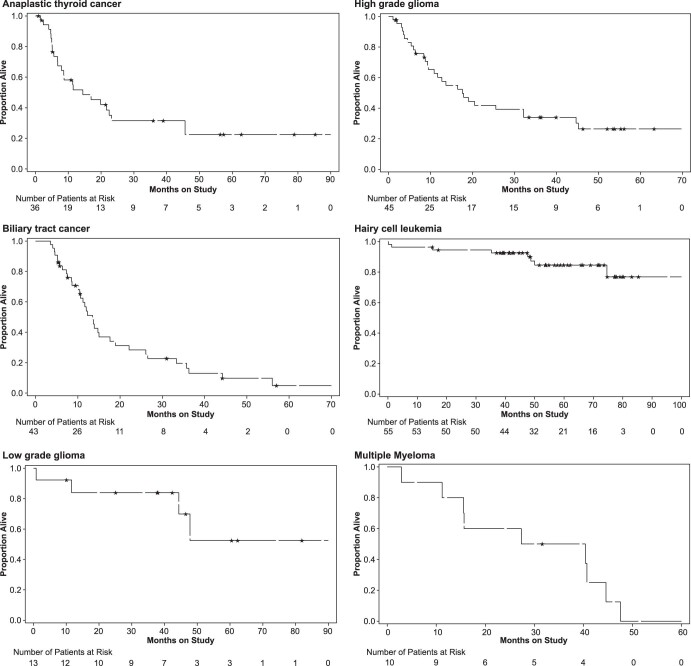
Extended Data Fig. 4. Overall survival across tumor cohorts.
Efficacy evaluable population: Primary and expansion cohorts in all except low grade glioma and multiple myeloma which are primary analysis cohorts. ATC: Among 36 patients, 24 (67%) patients died, 12 (33%) patients were censored due to end of follow-up. BTC: Among 43 patients, 34 (79%) patients died, nine (21%) patients were censored due to end of follow-up. ASI: All three patients died during the study LGG: Among 13 patients (in the primary analysis cohort), four (31%) patients died, nine (69%) patients were censored due to end of follow-up. HGG: Among 45 patients, 28 (62%) patients died, 17 (38%) patients were censored due to end of follow-up. HCL: Among 55 patients, eight (15%) patients died, 47 (85%) patients were censored due to end of follow-up. MM: Among 10 patients, nine (90%) patients died, one patient was censored due to end of follow up. ASI, adenocarcinoma of small intestine; ATC, anaplastic thyroid cancer; BTC, biliary tract cancer; G, grade; HCL, hairy cell leukemia; HGG, high (WHO G3/G4) grade glioma; LGG, low (WHO G1/G2) grade glioma; MM, multiple myeloma; WHO, World Health Organization.