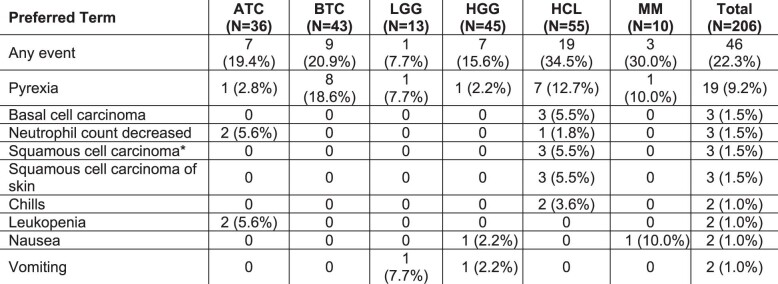
Extended Data Table 5.
SAEs (≥2 patients in total) suspected to be related to the study treatments
Values are number of patients, n (%).
Preferred terms by MedDRA version 23.0 and CTCAE version 4.0.
All treated patients (primary and expansion cohorts).
No suspected SAEs in the GIST and the ASI cohorts; hence, they are not included in the table.
Includes SAEs that are related to either dabrafenib or trametinib.
aAll three cases were cutaneous.
G, grade; MedDRA, Medical Dictionary for Regulatory Activities.