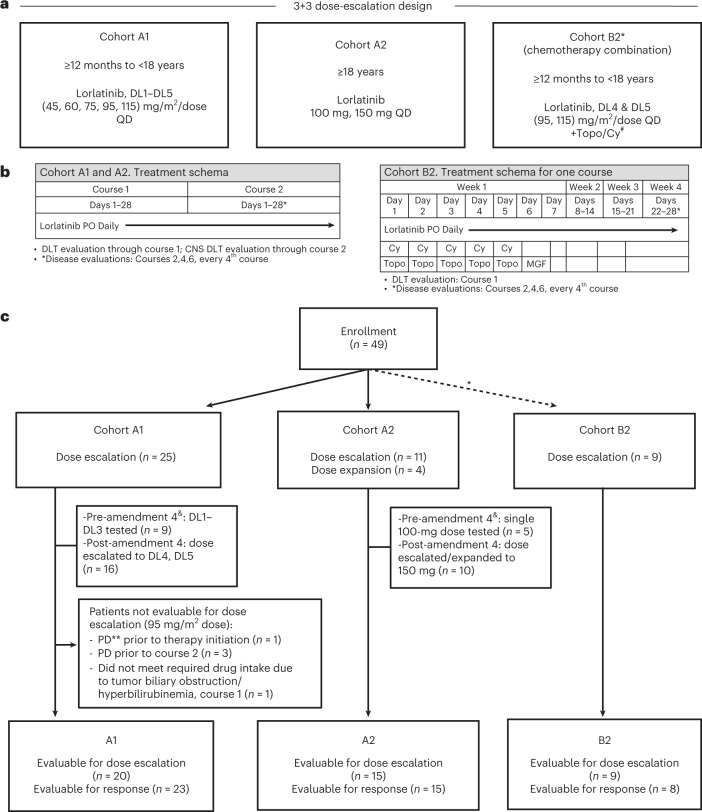
Fig. 1. Study design and patient disposition.
a, NANT 2015-02 study design. b, Treatment regimen by cohort. c, Patient distribution. *Cohort B2 opened at 95 mg/m2 once cohort A1, DL4 (95 mg/m2) was deemed tolerable and safe. B2 did not dose escalate to 115 mg/m2 until both B2, DL4 (95 mg/m2) in chemotherapy combination and cohort A1, DL5 (115 mg/m2) as single agent were deemed tolerable and safe. &Amendment 4: Additional doses of lorlatinib were added to dose-escalation cohorts A1 and A2 based on safety/PK data at the lower doses achieved, and pre-clinical data showing lorlatinib doses required for anti-neuroblastoma potency were higher than NSCLC pre-clinical models. #Topo/Cy, topotecan/cyclophosphamide; MGF, myeloid growth factor; **PD, progressive disease. PO, orally; QD, once a day.