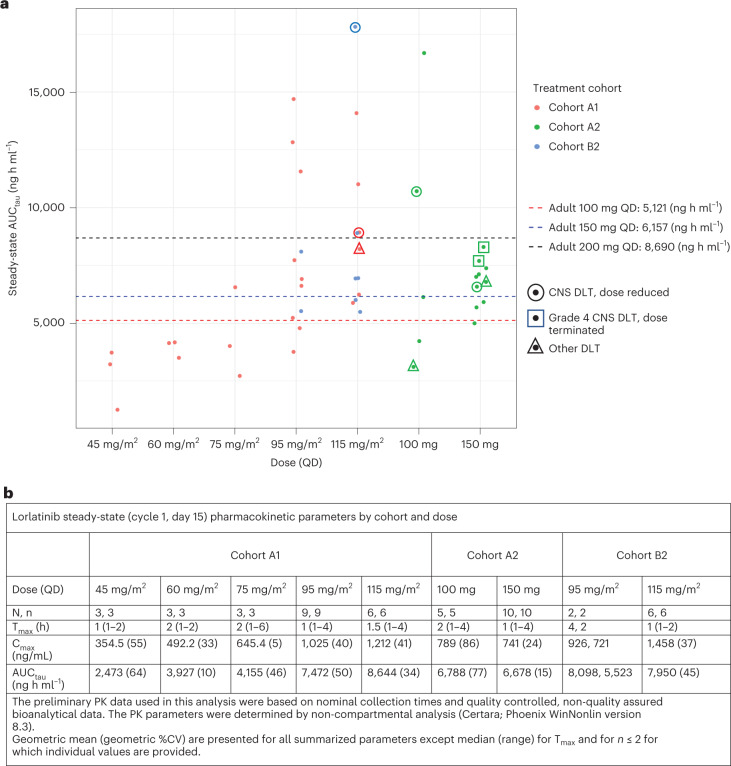
Fig. 2. PK analysis of lorlatinib exposure.
a, Lorlatinib AUCtau at steady state (cycle 1, day 15) compared to adult NSCLC levels. Dots represent individual patient AUCtau. Cohort A1 (<18 years), cohort A2 (>18 years) and cohort B2 (<18 years, lorlatinib in combination with topotecan/cyclophosphamide). b, Lorlatinib exposure at steady state summarized descriptively by cohort/dose. As shown, no differences in PK were observed for patients on B2 who received lorlatinib with chemotherapy. Although a patient on A1 with the highest Cmax and steady-state exposure for lorlatinib was a 12-year-old on DL5 who had a CNS DLT, there were six patients on A1 with steady-state exposures 1–2× higher than the adult 200-mg dose with no DLTs observed. CV, coefficient of variation; QD, once a day.