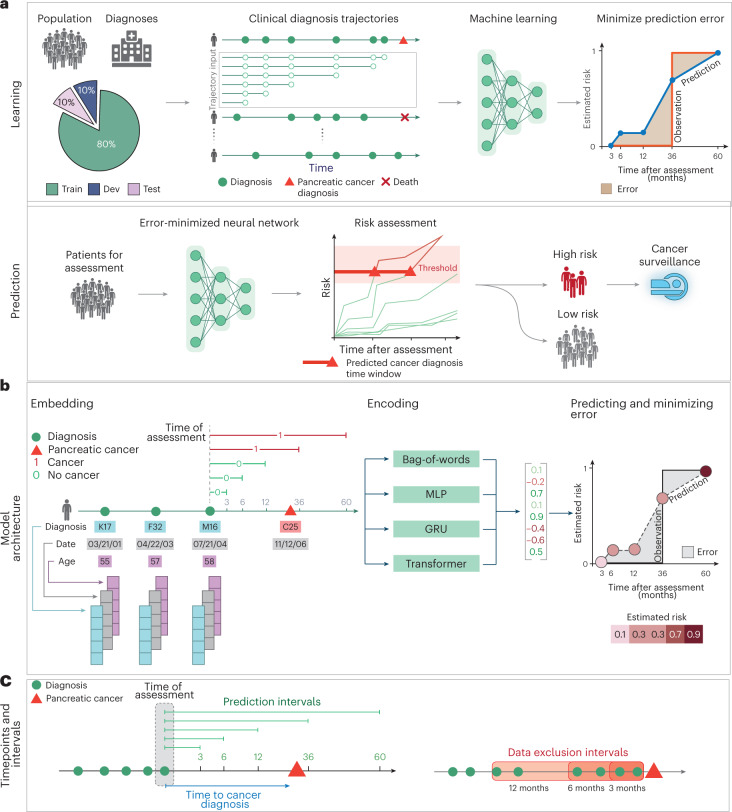
Fig. 1. Training and prediction of pancreatic cancer risk from disease trajectories.
a, Learning: The general ML workflow starts with partitioning the data into a training set (Train), a development set (Dev) and a test set (Test). The trajectories for training input are generated by sampling continuous subsequences of diagnoses for each patient’s diagnosis history, each starting with the first record but with different endpoints. The training and development sets are used for training so as to minimize the prediction error—that is, the difference between a risk score function (prediction) and a step function (observation), summed over all instances. Prediction: A model’s ability to accurately predict is evaluated using the withheld test set. The prediction model, depending on the prediction threshold selected from among possible operational points, discriminates between patients at higher and lower risk of pancreatic cancer. The risk model can guide the development of surveillance initiatives. b, The model trained with real-world clinical data has three steps: embedding, encoding and prediction. The embedding machine transforms categorical disease codes and timestamps of these disease codes into a lower-dimensional real number continuous space. The encoding machine extracts information from a disease history and summarizes each sequence in a characteristic fingerprint in the latent space (vertical vector). The prediction machine then uses the fingerprint to generate predictions for cancer occurrence within different time intervals after the time of assessment (3, 6, 12, 36 and 60 months). The model parameters are trained by minimizing the difference between the predicted and the observed cancer occurrence. c, Terminology for timepoints and intervals. The last event of a disease trajectory coincides with the time of assessment. From the time of assessment, cancer risk is assessed within 3, 6, 12, 36 and 60 months. To test the influence of close-to-cancer diagnosis codes on the prediction of cancer occurrence, exclusion intervals are used to remove diagnoses in the last 3, 6 and 12 months before cancer diagnosis.