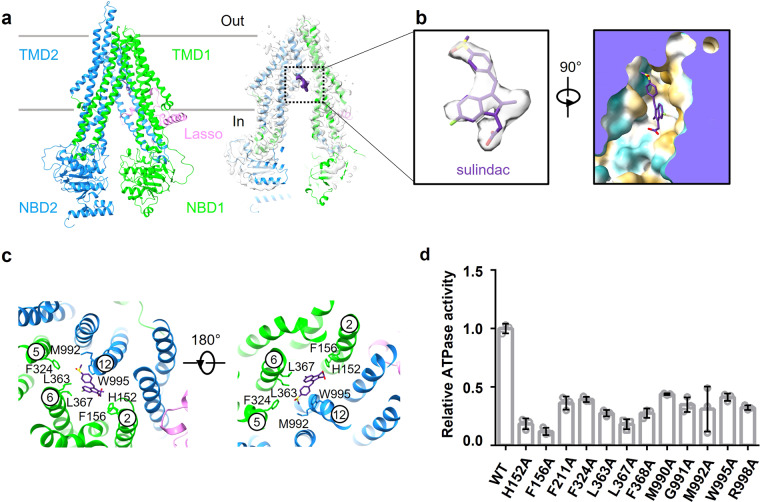
Fig. 3. Structural features of sulindac-bound hMRP4.
a Cartoon representation of the atomic model of sulindac-bound hMRP4 (left). Cryogenic electron microscopy map of sulindac-bound hMRP4 (right). b Electron microscopy density of sulindac in the same orientation as (A) (left). Sulindac is inserted into a pocket formed by the hydrophobic residues of hMRP4 (right). Contour level is 0.424. c Coordination of sulindac by hMRP4. Residues related to substrate binding are shown as sticks. Transmembrane helices (TMs) 2, 5, 6, and 12, which interact with sulindac, are labeled. d Relative ATPase activities of hMRP4 and mutants in a detergent of n-dodecyl-β-D-maltoside and cholesteryl hemisuccinate with 2 mM ATP in the absence of substrate (PGE1) or inhibitor (sulindac). Each data point is the mean of three independent experiments (n = 3), and the error bars represent the mean ± standard deviation. Source data are provided in Supplementary Data 3.