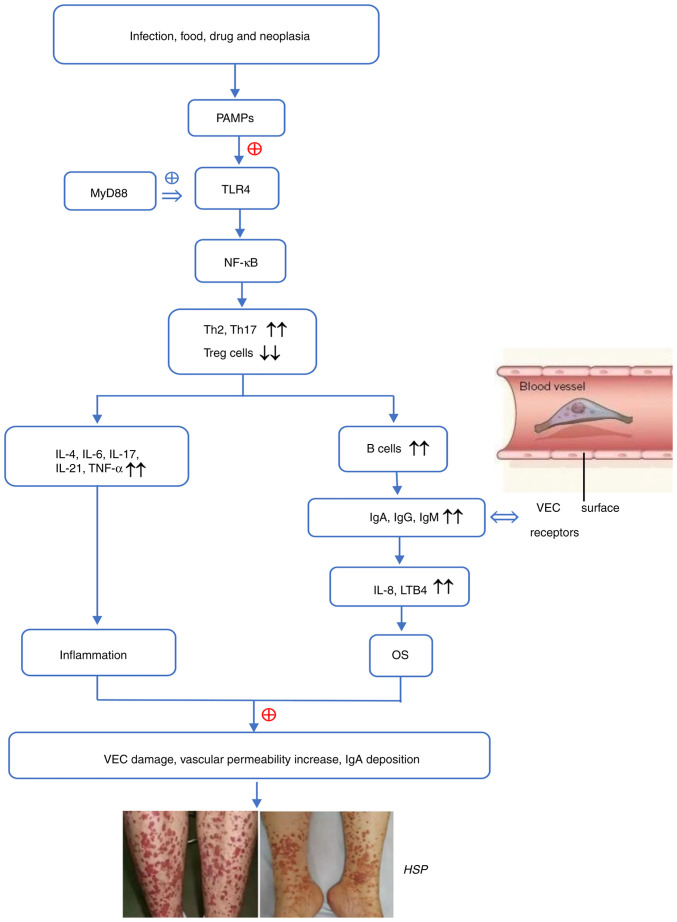
Figure 1.
The possible pathogenesis of HSP. PAMPs are specifically recognized by TLRs. By binding to MyD88, activated TLR4 stimulates the downstream signaling molecule NF-κB to trigger signal transduction cascades and activate immune cells. Th1/Th2 imbalance and overactivated Th17 release inflammatory cytokines and also accelerate the proliferation and differentiation of B cells, which secrete antibodies. IgA may combine with VEC surface receptors to produce IL-8 and LTB4, which recruit neutrophils to the blood vessel wall and promote OS and inflammation due to downregulation of superoxide dismutase, total antioxidant capacity, glutathione and heme oxygenase-1 and the upregulation of reactive oxygen species, malondialdehyde, nitric oxide and inflammatory factors. These alterations leads to VEC damage, an increase in vascular permeability and IgA being deposited on the vascular wall, thereby contributing to the occurrence and progression of HSP. HSP, Henoch-Schonlein purpura; OS, oxidative stress; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor κ-B; Th, T helper; IL, interleukin; TNF-α, tumor necrosis factor-α; Ig, immunoglobulin; LTB4, leukotriene B4; VEC, vascular endothelial cell; PAMP, pathogen-associated molecular pattern; Treg, regulatry T cell; ⊕, activation; ↔, binding; ↑↑, upregulation; ↓↓, downregulation.