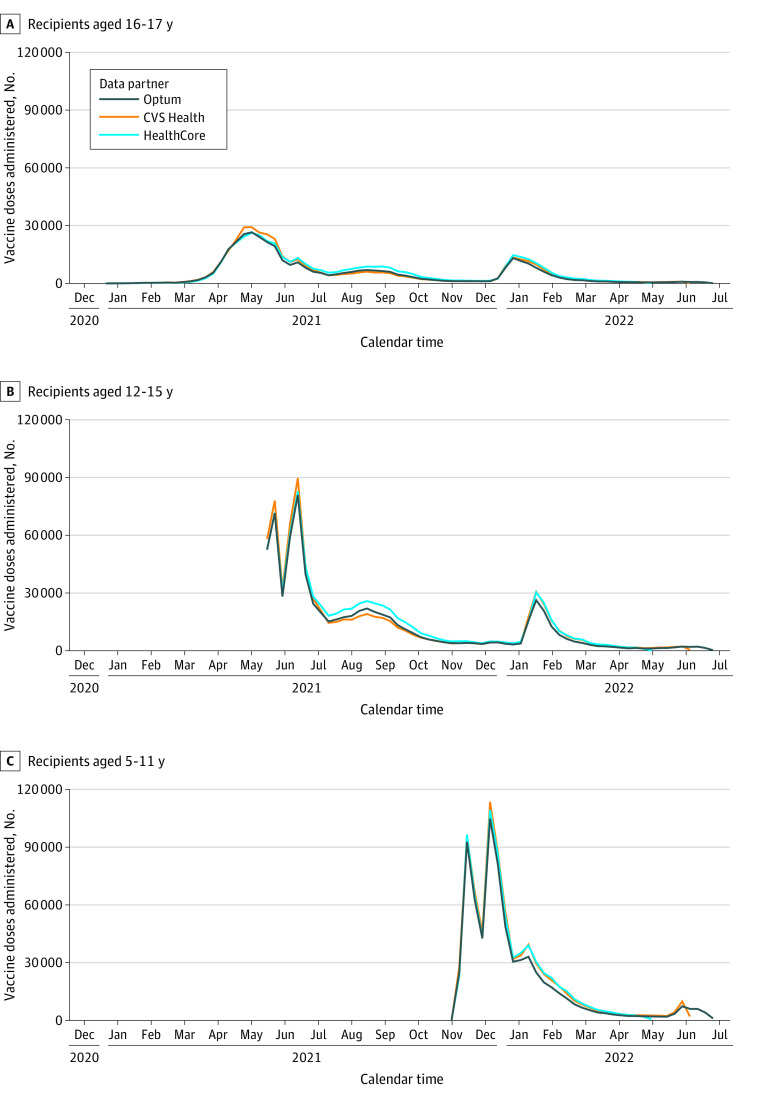
Figure. Vaccine Doses Administered Among Recipients Aged 5 to 17 Years With Data From the CVS Health, HealthCore, and Optum Databases.
The study start date was the earliest Emergency Use Authorization date for the BNT162b2 vaccination for each age group: for ages 5 to 11 years, October 29, 2021; ages 12 to 15 years, May 10, 2021; and ages 16 to 17 years, December 11, 2020. For the data cut dates, CVS Health data were available through May 31, 2022; HealthCore data through May 6, 2022; and Optum data through June 25, 2022.