Fig. 1.
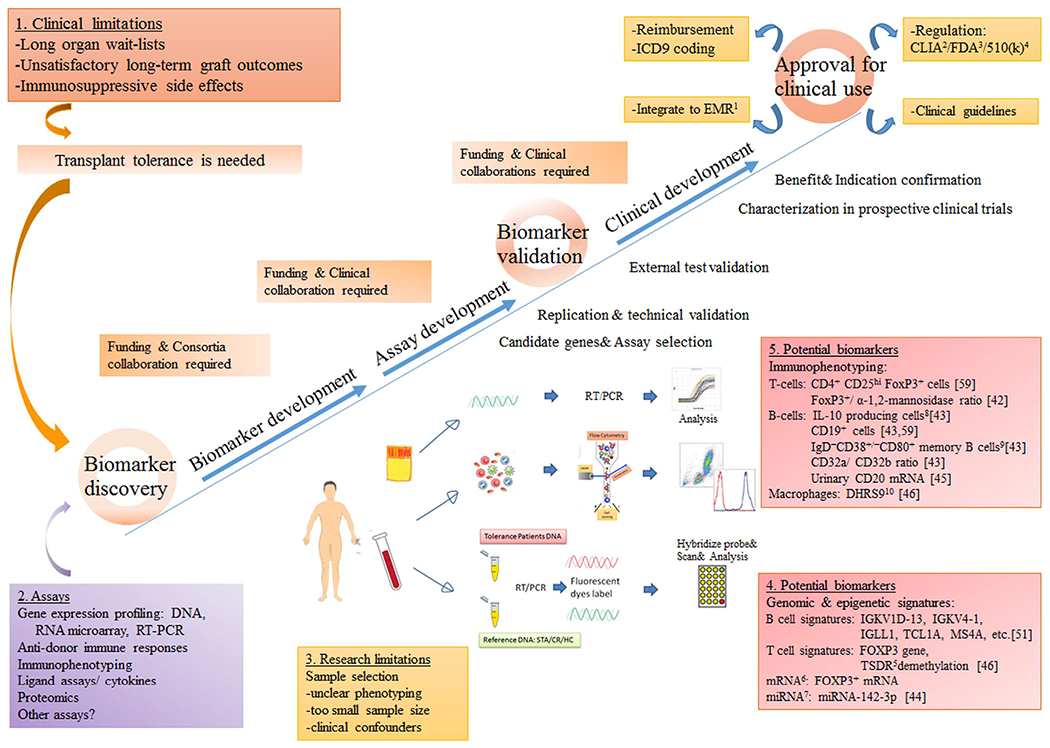
A summary of different components of successful biomarker discovery and validation for transplant tolerance. Abbreviation: 1EMR: electronic medical record, 2CLIA: The Clinical Laboratory Improvement Amendments, 3FDA: the Food and Drug Administration, 4510(k): section of the Food, Drug and Cosmetic Act requires device manufacturer who must register, to notify FDA of their intent to market a medical device at least 90 days in advance., 5TSDR: Regulatory T cells-specific demethylated region (TSDR), 6mRNA: messenger ribonucleic acid, 7miRNA: micro-ribonucleic acid, 8IL-10: Interleukin-10, 9IgD: immunoglobulin G, 10DHRS9: dehydrogenase/reductase 9. References numbers: provided in the brackets.
