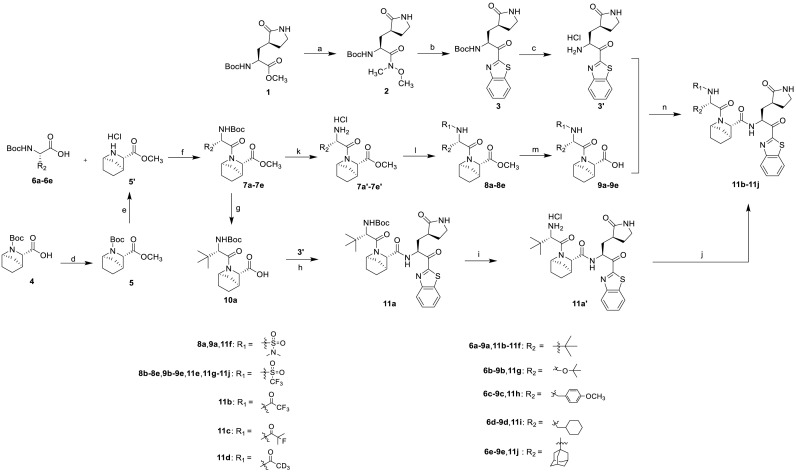
Scheme 1.
Synthesis of the target compounds 11a-11j. Reagents and conditions: (a) HN(OMe)Me·HCl, i-PrMgCl (2 M in THF), THF, 0 °C, 3 h; (b) benzothiazole, n-BuLi (1.6 M in THF), THF, −78 °C, 3 h; (c) 4 M HCl in 1, 4-dioxane, DCM, 20–25 °C, 40 min; (d) dimethyl sulfate, NaOH, THF, 65 °C, 2 h; (e) 4 M HCl in 1, 4-dioxane, DCM, 20–25 °C, 40 min; (f) HATU, DIEA, DCM, 20–25 °C, 3–5 h; (g) LiOH·H2O, THF, H2O, MeOH, 25 °C, 3 h; (h) HATU, DIEA, DCM/DMF, 20–25 °C, 3–5 h; (i) 4 M HCl in 1, 4-dioxane, DCM, 20–25 °C, 40 min; (j) trifluoroacetic anhydride/2-fluoroisobutyric acid/acetic hydride-d6/trifluoromethanesulfonic anhydride, Et3N, DCM, 20–25 °C, overnight; (k) 4 M HCl in 1, 4-dioxane, DCM, 20–25 °C, 40 min; (l) dimethylsulfamoyl chloride/trifluoromethanesulfonic anhydride, Et3N, DCM, 0 °C, 1 h, and then 25 °C, 1 h; (m) LiOH·H2O, THF, H2O, MeOH, 25 °C, 3 h; (n) HATU, DIEA, DCM/DMF, 20–25 °C, 3–5 h.