Abstract
Background:
Recurrence after resection of metastatic sarcoma is common. The gangliosides GM2, GD2 and GD3 are strongly expressed across sarcoma subtypes. We hypothesized that generation of anti-ganglioside antibodies would control micrometastases and improve outcomes in sarcoma patients who were disease-free after metastasectomy.
Methods:
We conducted a randomized phase II trial of the immunological adjuvant OPT-821 with a KLH-conjugated ganglioside vaccine targeting GM2, GD2 and GD3, versus OPT-821 alone in patients with metastatic sarcoma following complete metastasectomy. Patients received 10 subcutaneous injections at Weeks 1, 2, 3, 8, 16, 28, 40, 52, 68 and 84 and were followed for evidence of recurrent disease. The primary endpoint was relapse-free survival (RFS). Secondary endpoints included overall survival (OS) and serologic response.
Results:
A total of 136 patients were randomized, 68 to each arm. The mean age was 51.2, 52.2% were male, 90.4% had relapsed disease, 86.8% had high-grade tumors, and 14% had ≥ 4 metastases resected. Histologies included leiomyosarcoma (33%), spindle cell sarcoma (14%), undifferentiated pleomorphic sarcoma (13%), osteosarcoma (10%), synovial sarcoma (9%), liposarcoma (9%) and others (12%). Most adverse events (AEs) were Grade ≤ 2 (83.8% and 70.6% in the vaccine and adjuvant arms, respectively). The most common (≥ 20% of patients) were injection site reaction (89.7%), fatigue (44.1%), and pyrexia (27.9%) on the vaccine arm, and injection site reaction (69.1%) on the adjuvant only arm. The one-year RFS rate (34.5% and 34.8% in the vaccine and OPT-821 monotherapy arm, respectively) did not differ between arms (P = 0.725). One-year OS rates were 93.1% and 91.5% in the vaccine and OPT-821 monotherapy arm, respectively (P = 0.578). Serologic responses at week 9 were more frequent on the vaccine arm (96.5% of patients) than in the adjuvant arm (32.8%), and the difference between groups was durable.
Conclusions:
A sustained serologic response to vaccination was induced with the vaccine, but no difference in recurrence-free or overall survival was observed between treatment arms.
Keywords: Sarcoma, Adjuvant, Vaccine, Ganglioside
INTRODUCTION
Sarcomas are a heterogenous group of mesenchymal malignancies representing less than 1% of adult cancers.1 The prognosis of patients with metastatic sarcoma is poor, with a median overall survival of approximately 20 months from the time of metastasis in a recent study2, although prognosis is often histology-specific.3 The most frequent site of distant metastasis is the lung, followed by soft tissue, retroperitoneum, and other distant sites.4 Large retrospective studies have suggested that patients who undergo complete resection of distant metastases may have a higher survival rate than those with unresectable metastatic disease.5,6 Nevertheless, more than 50% of patients recur after pulmonary metastasectomy with an expected 5-year survival rate of 34%.7
Anti-cancer vaccination targeting tumor-associated antigens is an attractive strategy to activate the adaptive immune system and eliminate minimal residual disease.8 Gangliosides are a subclass of glycosphingolipids linked to sialic acids that are anchored to the cell membrane. They are often expressed in a range of sarcoma subtypes and are often immunogenic, triggering anti-ganglioside antibodies that are readily measurable in the peripheral blood.9–11 Eliciting an immune response to multiple gangliosides may lead to greater anti-neoplastic activity compared to a monovalent vaccination strategy.12 Further, conjugation of a ganglioside vaccine to keyhole limpet hemocyanin (KLH) induces a more potent T cell and antibody response.13,14
This randomized phase II study was designed to evaluate the hypothesis that administration of a trivalent vaccine targeting the GM2, GD2, and GD3 gangliosides, in addition to the novel immunological adjuvant OPT-821, would improve survival in patients with metastatic sarcoma rendered disease-free by surgery compared to treatment with OPT-821 alone. OPT-821 is a potent immunological adjuvant derived from the soapbark tree Quillaja saponaria that was found to be safe and immunogenic in the pre-clinical and clinical settings.15 As subcutaneous injection of OPT-821 consistently induces local induration and erythema when administered alone or in combination, it was required in the control arm to maintain double-blind status.
METHODS
This was a Phase II, randomized, double-blind, multi-center study of a trivalent ganglioside vaccine plus the immunological adjuvant OPT-821 versus OPT-821 alone for patients with metastatic sarcoma who were rendered disease-free (NCT01141491). The study was completed according to the Declaration of Helsinki and the international standards of Good Clinical Practice. The independent ethics committee or institutional review board of each participating study center approved the protocol and all amendments. All patients provided written informed consent.
Patient Selection
Eligible patients were ≥ 16 years of age with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and pathologic confirmation of newly diagnosed metastatic or distant relapsed sarcoma at their respective sarcoma center. Patient were required to be without radiological evidence of residual disease within 4 weeks of study initiation following surgery or multi-modality therapy. Non-surgical local ablative therapies, such as SRS or cryotherapy, were not acceptable as a replacement for surgical resection for the purpose of eligibility. Radiation or systemic chemotherapy must have been completed ≥ 4 weeks prior to treatment on this study and patients must have recovered from all adverse effects of treatment or have returned to baseline status. Adequate organ and marrow function at the time of enrollment was required.
Key exclusion criteria included: A diagnosis of Ewing sarcoma, embryonal rhabdomyosarcoma, and gastrointestinal stromal tumor histology, based on limited data for ganglioside expression in these tumor types. Patients with brain or bone metastases, retroperitoneal and/or uterine sarcoma with recurrent disease only in the abdomen or pelvis, and those whose disease recurred within 6 months of preceding treatment were also excluded. Patients on continuous doses of anti-inflammatory medications or those treated with a drug that had not received regulatory approval, and patients with any immunodeficiency disorders or illness, serologic positivity for Hepatitis B or C virus, or HIV were excluded.
Randomization and Blinding
During screening, patients were entered into an electronic data management system and randomization was accomplished by the method of random permuted blocks of four. Randomization was stratified by disease state at the time of enrollment (relapsed versus de novo metastasis at diagnosis). Patients presenting in the relapsed setting were further stratified by most recent disease-free interval (< 12 months versus ≥ 12 months) and number of relapses (1 versus > 1).
Study Procedures
Patients received a total of 10 injections, with one injection administered on visit weeks 1, 2, 3, 8, 16, 28, 40, 52, 68, and 84. Injections were administered subcutaneously into the upper arms, thighs or buttocks, rotating to different quadrants with each injection. Patients randomized to receive vaccine plus OPT-821 received a 1 mL subcutaneous injection containing 30 mcg of GM2, 30 mcg of GD2L, and 30 mcg of GD3L, each conjugated to KLH, plus 150 mcg of OPT-821. Patients randomized to OPT-821 only received a 1 mL subcutaneous injection containing150 mcg of OPT-821.
Patients were evaluated radiographically at Weeks 16, 28, 40, 52, 68, and 84, and every 12 weeks thereafter. Any clinical or radiographic evidence of recurrence was considered evidence of progression. If radiologic evidence of recurrence was found, pathologic confirmation was obtained whenever possible. If a biopsy was not possible and there were indeterminate radiographic findings, repeat imaging at a 4-week interval was permitted.
Adverse events (AEs) were assessed from the time of administration of the first vaccine through the end of the study (or death or recurrence of disease). Serology was collected at screening, prior to visit week 1, and at visit Weeks 9, 17, 40, 52, 68, 96 and 120.
Statistical Analyses
All statistical analysis utilized the intent-to-treat principle. All patients who received one or more injections of study treatment were included in the efficacy and safety analyses. In the efficacy analyses, patients were categorized by the arm to which they were randomized, regardless of the actual treatment received. For safety analyses, patients were categorized by the actual treatment received.
The primary endpoint of this study was relapse-free survival (RFS) in patients with pre-treatment antibody titer levels of < 1:20 against any one of the vaccine antigens (primary efficacy population). RFS was defined as time from randomization until any evidence of tumor growth or appearance of disease recurrence anywhere in the body, or death from any cause as determined by the principal investigator at each study center. The primary analysis was based upon a stratified log-rank test to compare the distributions of time to RFS. Patients without evidence of disease progression at the last follow-up were censored at that time point. Patients lost to follow-up for reasons related to disease progression were treated as progressing as of their last follow-up. The null hypothesis was that the distributions of time to RFS for the two arms are the same. The significance level for testing the null hypothesis was 20% (2-sided), a significance level suggested for preliminary studies of cancer vaccines.16
Secondary endpoints included: OS, defined as time of survival from randomization until death from any cause, efficacy analyses (RFS and OS) in the total population regardless of pre-treatment antibody levels, safety, and tolerability of the trivalent ganglioside vaccine plus OPT-821, as determined by reported AEs and changes in physical examinations and laboratory tests. Translational endpoints included evaluation of immune response to treatment, including correlating serologic response to vaccination with subsequent clinical outcome. In addition to the use of the log-rank test for comparing the distributions of time to event for RFS and OS, Kaplan-Meier curves characterized the event free rates over time.
A total of 134 patients were planned for this study, with 67 patients entered per treatment arm. The sample size was calculated assuming that 5% of patients would be excluded from the primary efficacy population because of high baseline antibody titers, a two–sided type 1 error rate of 20%, log-rank test (underlying exponential distribution), vaccine plus OPT-821 arm would have a 1-year RFS rate of 50%, the OPT-821 monotherapy arm would have a 1-year RFS of 30%, accrual would take 18 months, and a minimum of 12 months of follow-up per patient. With these assumptions and a sample size of 67 patients per treatment arm, the study would have 92% power to reject the null hypothesis. The primary analysis was scheduled when a total of 96 RFS events were recorded. The primary efficacy analysis was performed in August 2013, after 89 RFS events because of the unexpectedly slow event rate. Patients enrolled on the study were followed until November 2016, and the study closed in January 2017.
Continuous data were summarized with descriptive statistics of the mean, minimum, median, standard deviation, and number of patients. Counts and percentages summarized all categorical data. All statistical analyses were performed with the SAS® system (version 9.1.3 or later). All disposition, demographic, and baseline characteristics data were summarized and listed by treatment group and patient identifier.
The safety analysis included all patients enrolled in the study who received at least one injection of study treatment. Treatment emergent AEs were summarized by system organ class and preferred terms within a system organ class for each treatment group. AEs were coded using the MedDRA dictionary (version 11 or later).
RESULTS
Patient Characteristics
Between June 2010 and December 2012, a total of 136 patients were randomized on this study, 68 to each treatment arm. Patient characteristics (Table 1) were generally well balanced between randomization arms. Most study patients were < 65 years of age (83.8% in the vaccine arm and 75% in the OPT-821 monotherapy arm). Approximately half of patients had a recent disease-free interval of < 12 months and had received prior radiation therapy or chemotherapy. Most patients on the vaccine and adjuvant alone arms, respectively, had relapsed (n = 62 [91%] vs. 61 [90%]) high-grade (62 [91%] vs. 56 [82%]) soft tissue disease (35 [52%] vs. 32 [47%]) that was localized at the time of diagnosis (55 [81%] vs. 57 [84%]). Forty-one patients (31%) completed all 10 of the planned vaccine injections.
Table 1:
Patient characteristics
| Vaccine + OPT-821 (N=68) | OPT-821 (N=68) | |
|---|---|---|
| Age (years) | ||
| n | 68 | 68 |
| Mean (SD) | 49.8 (13.25) | 52.5 (15.24) |
| Sex - n (%) | ||
| Male | 39 (57.4) | 32 (47.1) |
| Female | 29 (42.6) | 36 (52.9) |
| Race - n (%) | ||
| American Indian or Alaska Native | 1 (1.5) | 0 |
| Asian | 4 (5.9) | 3 (4.4) |
| Black or African American | 1 (1.5) | 3 (4.4) |
| White | 62 (91.2) | 61 (89.7) |
| Other | 0 | 1 (1.5) |
| Ethnicity - n (%) | ||
| Hispanic or Latino | 3 (4.4) | 2 (2.9) |
| Not Hispanic or Latino | 65 (95.6) | 66 (97.1) |
| ECOG Performance Status– n (%) | ||
| 0 | 57 (83.8) | 48 (70.6) |
| 1 | 11 (16.2) | 20 (29.4) |
| Number of Metastases – n (%) | ||
| 1 to 3 | 60 (88.2) | 57 (83.8) |
| 4 or more | 8 (11.8) | 11 (16.2) |
| Primary Tumor Grade - n (%) | ||
| High | 62 (91.2) | 56 (82.4) |
| Intermediate | 5 (7.4) | 9 (13.2) |
| Low | 1 (1.5) | 3 (4.4) |
| Primary Site of Original Disease – n (%) | ||
| Soft Tissue | 35 (51.5%) | 32 (47.1%) |
| Other | 18 (26.5%) | 20 (29.4%) |
| Bone | 8 (11.8%) | 6 (8.8%) |
| Abdomen | 4 (5.9%) | 7 (10.3%) |
| Lungs | 1 (1.5%) | 2 (2.9%) |
| Kidney | 1 (1.5%) | 1 (1.5%) |
| Head and Neck | 1 (1.5%) | 0 |
| Metastatic Disease at time of Original Diagnosis – n (%) | ||
| Yes | 13 (19.1%) | 11 (16.2%) |
| No | 55 (80.9%) | 57 (83.8%) |
| Most Recent Disease-free Interval – n (%) | ||
| < 12 months | 34 (50.0%) | 33 (48.5%) |
| >= 12 months | 28 (41.2%) | 28 (41.2%) |
| Number of Relapses – n (%) | ||
| 1 | 34 (50.0%) | 36 (52.9%) |
| > 1 | 28 (41.2%) | 25 (36.8%) |
| Prior Radiotherapy - n (%) | ||
| Yes | 35 (51.5%) | 34 (50.0%) |
| No | 33 (48.5%) | 34 (50.0%) |
| Prior Chemotherapy – n (%) | ||
| Yes | 35 (51.5%) | 37 (54.4%) |
| No | 33 (48.5%) | 31 (45.6%) |
| Tumor Histology - n (%) | ||
| Leiomyosarcoma (n=45) | 17 (25%) | 28 (41%) |
| Spindle Cell Sarcoma (n=19) | 12 (18%) | 7 (10%) |
| Pleomorphic Sarcoma (n=18) | 9 (13%) | 9 (13%) |
| Osteosarcoma (n=14) | 7 (10%) | 7 (10%) |
| Liposarcoma (n=12) | 6 (9%) | 6 (9%) |
| Synovial Sarcoma (n=12) | 5 (7%) | 7 (10%) |
| Other (n=16) | 12 (18%) | 4 (6%) |
Safety
Overall, study treatment was well tolerated across both arms. Sixty-six (97.1%) patients on the vaccine arm and 60 (88.2%) on the OPT-821 monotherapy arm experienced an AE. The most common AEs on the vaccine arm were injection site reaction (89.7%), fatigue (44.1%), pyrexia (27.9%), injection site erythema (17.6%), and influenza-like illness (14.7%). On the adjuvant alone arm, the most frequent AEs were injection site reaction (69.1%), fatigue (19.1%), diarrhea (13.2%), and injection site pain (11.8%) (Table 2). Most AEs were Grade ≤ 2 (83.8% and 70.6% in the vaccine and adjuvant arms, respectively). Grade ≥ 3 AEs occurred in 9 patients (13.2%) in the vaccine arm and 12 patients (17.6%) on the adjuvant alone arm. There was one patient death on the vaccine arm (the cause of death was unknown) that was deemed possibly related to study treatment. Treatment emergent serious AEs occurred in 4 patients (5.9%) on the vaccine arm and 7 (10.3%) in OPT-821 monotherapy arm (Supplemental Table S1). One patient on the adjuvant alone arm (generalized rash) and three on the vaccine arm (maculopapular rash, Grade 1–2 anaphylactoid reaction, and death) discontinued treatment due to an adverse event.
Table 2:
Adverse events occurring in ≥ 5% of the study population
| AE by system organ class preferred term* | Vaccine plus OPT-821 | OPT-821 | ||
|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | |
| Gastrointestinal disorders | ||||
| Abdominal pain | 4 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 2 (2.9) | 0 (0.0) | 9 (13.2) | 0 (0.0) |
| Nausea | 5 (7.4) | 1 (1.5) | 6 (8.8) | 0 (0.0) |
| General disorders and administration site conditions | ||||
| Fatigue | 30 (44.1) | 0 (0.0) | 13 (19.1) | 0 (0.0) |
| Influenza like illness | 10 (14.7) | 0 (0.0) | 3 (4.4) | 0 (0.0) |
| Injection site erythema | 12 (17.6) | 0 (0.0) | 5 (7.4) | 0 (0.0) |
| Injection site oedema | 8 (11.8) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| Injection site pain | 8 (11.8) | 0 (0.0) | 8 (11.8) | 0 (0.0) |
| Injection site reaction | 59 (86.8) | 2 (2.9) | 47 (69.1) | 0 (0.0) |
| Malaise | 5 (7.4) | 0 (0.0) | 3 (4.4) | 0 (0.0) |
| Pain | 5 (7.4) | 0 (0.0) | 3 (4.4) | 0 (0.0) |
| Pyrexia | 18 (26.5) | 1 (1.5) | 2 (2.9) | 0 (0.0) |
| Infections and infestations | ||||
| Upper respiratory tract infection | 8 (11.8) | 0 (0.0) | 7 (10.3) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 4 (5.9) | 0 (0.0) | 5 (7.4) | 0 (0.0) |
| Myalgia | 4 (5.9) | 0 (0.0) | 4 (5.9) | 0 (0.0) |
| Pain in extremity | 4 (5.9) | 0 (0.0) | 6 (8.8) | 0 (0.0) |
| Nervous system disorders | ||||
| Headache | 6 (8.8) | 0 (0.0) | 6 (8.8) | 1 (1.5) |
| Psychiatric disorders | ||||
| Anxiety | 6 (8.8) | 0 (0.0) | 6 (8.8) | 0 (0.0) |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 8 (11.8) | 0 (0.0) | 3 (4.4) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | ||||
| Erythema | 4 (5.9) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Rash | 5 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
All numbers represent number of patients (%)
Efficacy
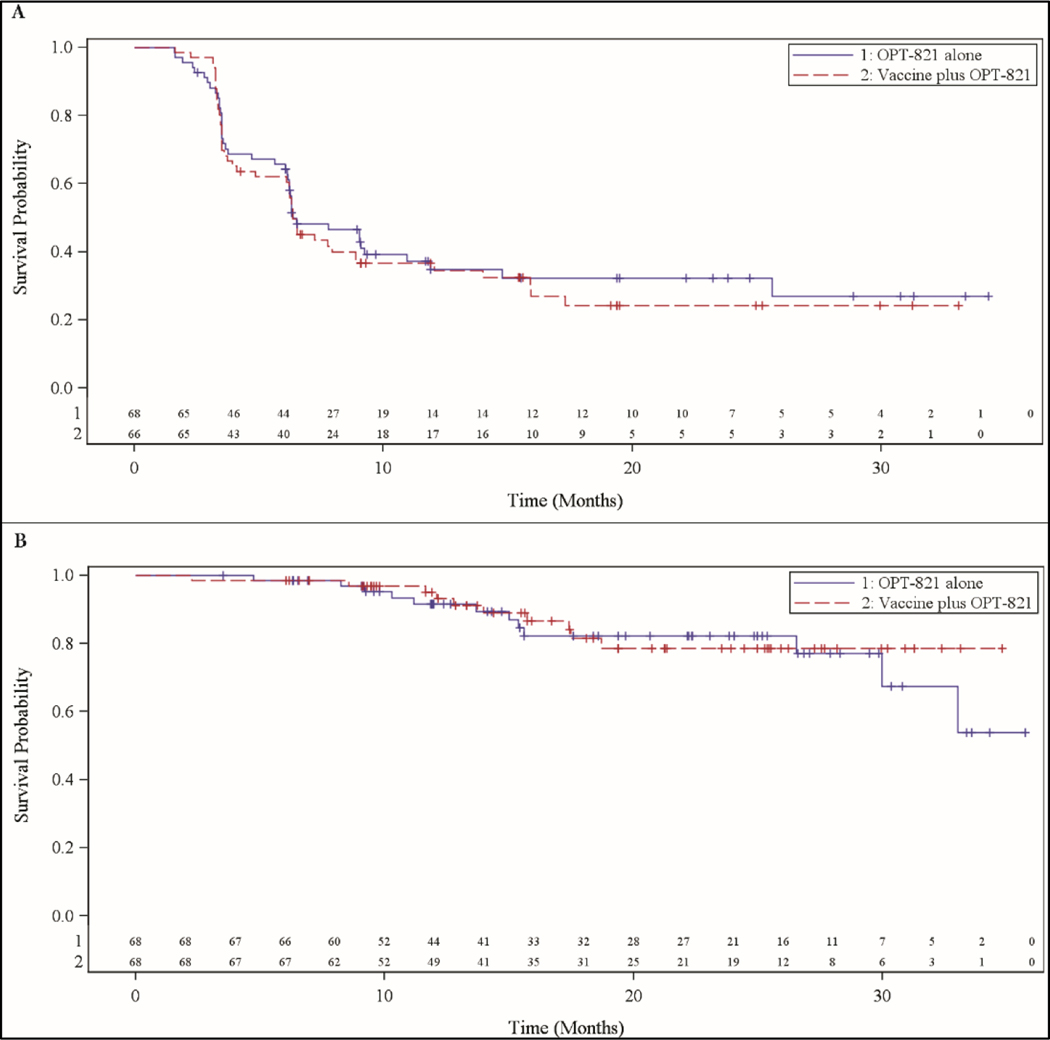
All patients enrolled and treated were included in the primary efficacy population. The mean (standard deviation) number of vaccine administrations was 6.2 (2.35) in the vaccine arm and 6.2 (2.23) in the adjuvant alone arm. The primary efficacy analysis was performed 8 months after enrollment of the last patient and 39 months after enrollment of the first patient. Progression events had occurred in 46 patients in the vaccine arm and 43 in the OPT-821 monotherapy arm. The RFS rate at one year was 34.5% in the vaccine arm and 34.8% in the OPT-821 monotherapy arm (P = 0.725). The median relapse-free survival was 191 days in each arm (95% confidence interval [CI] 104 – 420 in the vaccine arm and 141 – 357 in the control) (Figure 1A). Ten patients in the vaccine arm and 12 in the OPT-821 monotherapy arm died. The 1-year OS rates were 93.1% and 91.5% in the vaccine and OPT-821 monotherapy arm, respectively (P = 0.578). Median overall survival was not reached in either treatment arm (Figure 1B).
Figure 1:
Kaplan-Meier curves of (A) RFS, (B) OS performed from the primary efficacy analysis data 3.2 years after trial initiation (8 months after accrual of final patient)
Serologic Response
At baseline, 32% of patients (22 of 68) who received the vaccine and 22% (14 of 64) who received the adjuvant alone had a detectable serologic response (defined as any anti-ganglioside antibody titer of > 1:20) to one of the vaccine antigens. At week 9, serologic responses were more frequent in the vaccine arm (96.5%; 55 of 57) than in the adjuvant arm (32.8%; 21 of 64). On the adjuvant only arm, 9.4% of patients (6 of 64) had an increase of their titers with treatment compared to 93% (53 of 57) on the vaccine arm. At the last time point (weeks 40 – 68), the differences in serologic response remained apparent (89.3% [25/45] of patients on the vaccine arm vs. 20.8% on the adjuvant only [4/44]) (Table 3).
Table 3:
Overall serologic response to study treatment
| Vaccine + OPT-821 (N=68) | OPT-821 (N=68) | |
|---|---|---|
| Baseline - n (%) | ||
| High | 3 (4.4) | 3 (4.7) |
| Low | 19 (27.9) | 11 (17.2) |
| Negative | 46 (67.6) | 50 (78.1) |
| Total | 68 | 64 |
| Week 9 - n (%) | ||
| High | 42 (73.7) | 3 (4.7) |
| Low | 13 (22.8) | 18 (28.1) |
| Negative | 2 (3.5) | 43 (67.2) |
| Total | 57 | 64 |
| Week 17 - n (%) | ||
| High | 31 (68.9) | 3 (6.8) |
| Low | 13 (28.9) | 12 (27.3) |
| Negative | 1 (2.2) | 29 (65.9) |
| Total | 45 | 44 |
| Week 40 - 68 - n (%) | ||
| High | 17 (58.6) | 2 (8.3) |
| Low | 10 (34.5) | 4 (16.7) |
| Negative | 2 (6.9) | 18 (75.0) |
| Total | 29 | 24 |
High titers: > 1:160, low: > 1:20 – 1:160, negative: ≤ 1:20
DISCUSSION
To our knowledge, this randomized, double-blind, controlled trial is the largest vaccine trial of its kind in sarcoma patients. The study did not meet its primary endpoint. No significant difference in RFS was seen between patients who received a trivalent KLH-conjugated ganglioside vaccine administered with the adjuvant OPT-821 compared to patients who received OPT-821 alone. There was a lack of efficacy in the vaccine arm above and beyond the control despite measurable and durable serologic responses seen across antibody class (IgM and IgG) and ganglioside target (GM2, GD2, and GD3). However, both the trivalent ganglioside vaccine and the adjuvant OPT-821 were safe and tolerable. AEs were generally mild and as expected for a subcutaneously injected vaccine, with injection site reactions or fatigue being the most common treatment related TEAEs on the vaccine arm.
A key strength of this study is its large size for a prospective interventional trial in a rare group of diseases. It sheds light on the natural history of sarcoma patients who underwent metastasectomy and were rendered free of disease. The time to progression in each treatment arm of this study was approximately 6 months, while the 1-year RFS rate was 35%. These data are in line with prior retrospective reports, in which the median time to recurrence after initial metastasectomy was 6.8 months.7 Longer term survival data would be of interest, but is not available from the study sponsor MabVax Therapeutics, which is no longer in operation. Subgroup analysis by tumor histology was previously presented at the American Society of Clinical Oncology Annual Meeting, showing no difference in RFS when stratified by histology.17
The reason why the trivalent ganglioside vaccine did not prolong survival in this study population is not clear. Anti-GD2 monoclonal antibodies are now standard of care in the treatment of high-risk neuroblastoma in combination with interleukin-2 and/or granulocyte-macrophage colony-stimulating factor (GM-CSF), serving as proof-of-principle in targeting these antigens with antibody therapy.18,19 However, a recent phase II study of dinatuximab plus GM-CSF in recurrent osteosarcoma rendered disease-free by surgery, a disease known to express high levels of GD220, failed to reach its primary endpoint of event-free survival.21 These results suggest that anti-GD2 antibody monotherapy is ineffective in osteosarcoma. Similarly, the results of our study imply that dinatuximab alone would not be effective in other sarcoma subtypes, as anti-ganglioside antibody titers generated by the trivalent vaccine did not lead to improved clinical outcomes. However, a formal comparison between the quantity and quality of anti-GD2 antibodies in the dinatuximab studies and the vaccine-induced antibodies in our study is not possible. It is also notable that ganglioside expression was not measured in either the osteosarcoma-specific dinatuximab trial or in this study utilizing a trivalent ganglioside vaccine. It is possible that ganglioside expression is lost in advanced sarcomas, in which case ganglioside-targeted therapies would be futile.
Even if ganglioside expression is retained in advanced sarcomas, anti-ganglioside antibodies titers generated by the vaccine may not have been high enough or had high enough affinity to elicit clinically meaningful activity, a possibility that could theoretically be addressed by utilizing anti-ganglioside monoclonal antibodies with higher affinity. Alternatively, circulating antibodies alone may be insufficient to mount anti-tumor activity in sarcomas without activation of T cells. Other mechanisms may contribute to ganglioside vaccine resistance in sarcomas, such as upregulation of immunosuppressive myeloid-derived suppressor cells, heterogeneity of ganglioside expression in the tumor, downregulation of antigen-presentation machinery, or upregulation of inhibitor immune checkpoint molecules after vaccination.22–24 Although this trial was initiated before the advent of anti-PD-(L)1 therapy across cancer types, there is increased interest today in combining immune checkpoint blockade with therapeutic vaccines to elicit a more robust immune response.25,26 Recently reported data in high-risk neuroblastoma suggests that adding an oral yeast β-glucan as an adjuvant to a multi-valent ganglioside vaccine conjugated to KLH can successfully boost antibody-dependent cell-mediated cytotoxicity.19,27 GD2-directed chimeric antigen receptor (CAR) T cells have also demonstrated promise in very early clinical trials in both neuroblastoma and H3K27M-mutated diffuse midline gliomas.28,29 Thus, gangliosides such as GD2 are still promising targets in sarcoma, but novel strategies are likely needed to target them or to augment the anti-tumor activity of anti-ganglioside antibodies.
This trial has several limitations. It enrolled multiple histologic subtypes of sarcoma, which are now increasingly recognized as unique disease entities with their own natural histories and immune profile, which could make finding a signal of efficacy more difficult. Patients were not screened for or stratified by ganglioside expression, and GM2, GD2, and GD3 may not truly be ‘shared’ antigens across all tumors. Nevertheless, gangliosides are still attractive therapeutic targets. Future research should focus on confirming target engagement with an anti-ganglioside therapy and measuring immune activation or anti-tumor activity, above and beyond serological response.
Supplementary Material
Highlights.
Sarcomas overexpress gangliosides, which are attractive therapeutic targets
In this trial, a tri-valent ganglioside vaccine failed to prolong relapse-free survival
The vaccine was generally safe, well tolerated, and generated a serologic response
New strategies are needed to elicit a clinically meaningful anti-ganglioside immune response
ACKNOWLEDGEMENTS
We would like to recognize the patients and their families for participating in this research study and the Memorial Sloan Kettering Clinical Grade Production (CGP) Facility for Vaccine Production, which was partially supported by NCI Cancer Center Support grant P30 CA008748.
FUNDING
MabVax Therapeutics provided funding for this study.
Footnotes
Clinicaltrials.gov identifier: NCT01141491
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations: ASCO 2013, ASCO 2014
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE & Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 71, 7–33, doi: 10.3322/caac.21654 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Tap WD et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 323, 1266–1276, doi: 10.1001/jama.2020.1707 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan MC et al. Histology-based Classification Predicts Pattern of Recurrence and Improves Risk Stratification in Primary Retroperitoneal Sarcoma. Ann Surg 263, 593–600, doi: 10.1097/SLA.0000000000001149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billingsley KG et al. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer 85, 389–395 (1999). [PubMed] [Google Scholar]
- 5.Billingsley KG et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 229, 602–610; discussion 610–602 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jablons D. et al. Metastasectomy for soft tissue sarcoma. Further evidence for efficacy and prognostic indicators. The Journal of thoracic and cardiovascular surgery 97, 695–705 (1989). [PubMed] [Google Scholar]
- 7.Chudgar NP et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. The Journal of thoracic and cardiovascular surgery 154, 319–330 e311, doi: 10.1016/j.jtcvs.2017.02.061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena M, van der Burg SH, Melief CJM & Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer 21, 360–378, doi: 10.1038/s41568-021-00346-0 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Chang HR, Cordon-Cardo C, Houghton AN, Cheung NK & Brennan MF Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer 70, 633–638 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY & Cheung NK Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer 63, 1780–1785, doi: 10.1002/pbc.26097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez CA et al. Serum anti-ganglioside IgM antibodies in soft tissue sarcoma: clinical prognostic implications. Cancer J 8, 384–394, doi: 10.1097/00130404-200209000-00009 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Chapman PB et al. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-keyhole limpet hemocyanin + QS21 vaccine: a dose-response study. Clin Cancer Res 6, 874–879 (2000). [PubMed] [Google Scholar]
- 13.Helling F. et al. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res 54, 197–203 (1994). [PubMed] [Google Scholar]
- 14.Helling F. et al. GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res 55, 2783–2788 (1995). [PubMed] [Google Scholar]
- 15.Kushner BH et al. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res 20, 1375–1382, doi: 10.1158/1078-0432.CCR-13-1012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn EL et al. Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Oncol 19, 265–272, doi: 10.1200/JCO.2001.19.1.265 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Carvajal RD et al. Trivalent ganglioside vaccine and immunologic adjuvant versus adjuvant alone in metastatic sarcoma patients rendered disease-free by surgery: A randomized phase 2 trial. Journal of Clinical Oncology 32, 10520–10520, doi: 10.1200/jco.2014.32.15_suppl.10520 (2014). [DOI] [Google Scholar]
- 18.Yu AL et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363, 1324–1334, doi: 10.1056/NEJMoa0911123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung IY et al. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression. J Clin Oncol 39, 215–226, doi: 10.1200/JCO.20.01892 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon VI et al. Ganglioside GD2 expression is maintained upon recurrence in patients with osteosarcoma. Clin Sarcoma Res 5, 4, doi: 10.1186/s13569-014-0020-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hingorani P. et al. Phase 2 study of anti-disialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma: A report from the Children’s Oncology Group. Eur J Cancer 172, 264–275, doi: 10.1016/j.ejca.2022.05.035 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iclozan C, Antonia S, Chiappori A, Chen DT & Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 62, 909–918, doi: 10.1007/s00262-013-1396-8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carretero R. et al. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int J Cancer 129, 839–846, doi: 10.1002/ijc.25733 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Casper ES et al. Biological study of R24 mouse monoclonal antibody in patients undergoing thoracotomy for pulmonary metastases from soft tissue sarcoma. Cancer Invest 12, 20–25, doi: 10.3109/07357909409021389 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Ott PA et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 183, 347–362 e324, doi: 10.1016/j.cell.2020.08.053 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Ott PA et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221, doi: 10.1038/nature22991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung NK, Modak S, Vickers A. & Knuckles B. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol Immunother 51, 557–564, doi: 10.1007/s00262-002-0321-3 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majzner RG et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941, doi: 10.1038/s41586-022-04489-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heczey A. et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med 26, 1686–1690, doi: 10.1038/s41591-020-1074-2 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.