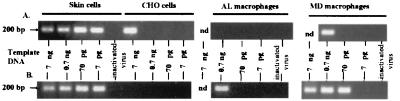
FIG. 6.
In vitro infection of afferent lymph and monocyte derived macrophages. CD14hi CD1b−/lo macrophages were separated by FACS from afferent lymph (AL macrophages), and macrophages were derived in culture from ovine peripheral blood monocytes (MD macrophages). Skin and CHO cells were harvested from stocks in culture. Cells were infected with DNase-treated MVV (0.06 TCID50/cell) for 2 h, and then additional medium was added for overnight (A) incubation or a 48-h (B) incubation. DNA was extracted from the cells, quantified with a PicoGreen dsDNA quantitation reagent kit, and used as template at the given amounts (7 ng to 7 pg) in a nested PCR. Heat-inactivated virus was noninfectious, as determined by cocultivation, and was used at preinactivation titers as above. For these controls, 7 ng (skin cells and CHO cells) or 0.7 ng (AL macrophages and MD macrophages) of template DNA was used. The expected size for the PCR-amplified early reverse transcription product of EV1 MVV is 203 bp. This experiment is representative of four experiments using two different sheep. FACS-separated afferent lymph macrophages were 95.8% pure after deduction of backgrounds for autofluorescence and spectral overlap. nd, not done.