Fig. 1: In vitro screening of human specific IgE mAbs for peanut allergen Ara h 2 epitope specificity.
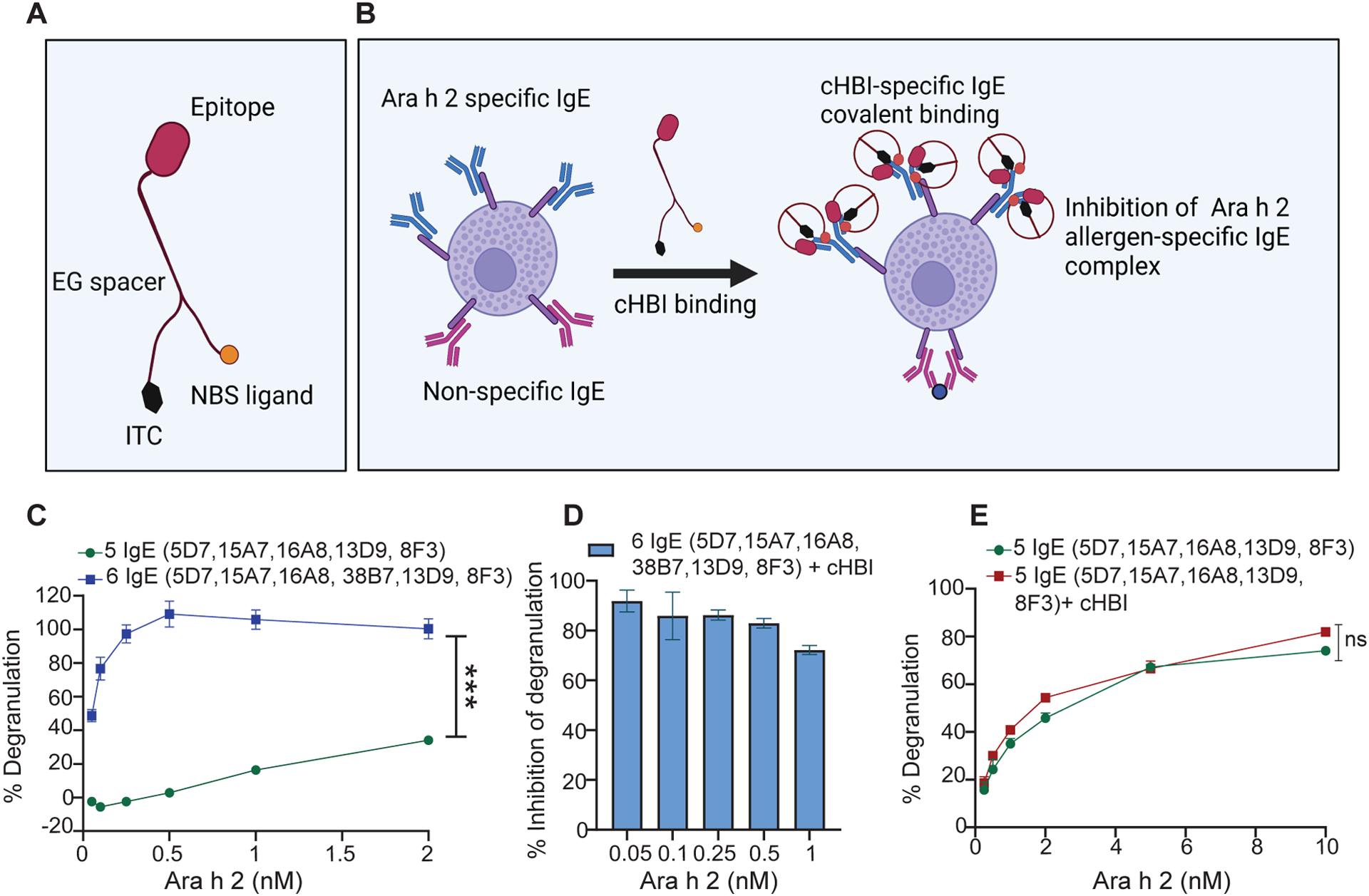
(A) Illustration of cHBI structure shows the antigen binding site (peptide epitope), nucleotide binding site (NBS), and isothiocyanate moiety (ITC). (B) Schematic illustration of cHBI mechanism of action. cHBI binds to Ara h 2 specific IgE on mast cells forming an irreversible covalent bond and preventing the binding of Ara h 2 allergen to specific IgE inhibiting the allergic reactions. (C) Line graph of percent RBL-SX38 cell degranulation. RBL-SX38 cells primed with two cocktails of IgE mAbs targeting different epitopes of Ara h 2 allergen. One cocktail with all six IgE mAbs, and one cocktail with 5 IgE mAbs without clone 38B7 mAb. The cells were challenged with Ara h 2 at (0.05, 0.10, 0.25, 0.5, 1.0, 2.0 μM) 24 hours post IgE priming. (D) Percent inhibition of RBL-SX38 cell degranulation by cHBI when RBL-SX38 cells primed with a cocktail of six IgE mAbs and challenged with Ara h 2 at (0.05, 0.10, 0.25, 0.5, 1.0 μM). (E) Line graph of percent RBL-SX38 cell degranulation. RBL-SX38 cells primed with a cocktail of 5 specific IgE mAbs without clone 38B7 mAb in the presence or absence of cHBI and then challenged with Ara h 2 at (0.05, 0.10, 0.25, 0.5, 1.0, 2.0, 5.0, 10.0 μM). Data represent mean ±SEM, mean in each graph represents three technical replicates. Data are representative of two independent experiments. One way ANOVA with t-test was used to compare means in (C), and (E). ***P <0.001. ns indicates no statistical difference. Illustrations in (A) and (B) created with BioRender.com.
