Table 3.
SAR exploration of substituent groups at the N3 position of quinazolin-4-onea.
| Compd | R3 | IC50 (μM) |
|---|---|---|
| C1 | 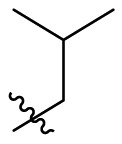 |
0.124 ± 0.018 |
| C2 | 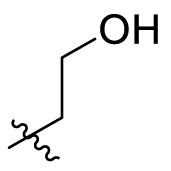 |
1.365 ± 0.062 |
| C3 | 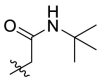 |
0.949 ± 0.077 |
| C4 | 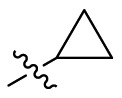 |
0.290 ± 0.028 |
| C5 | 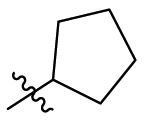 |
0.124 ± 0.016 |
| C6 | 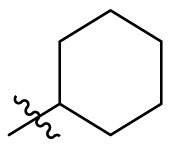 |
0.274 ± 0.022 |
| C7 | 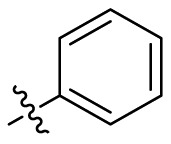 |
0.083 ± 0.006 |
| C8 | 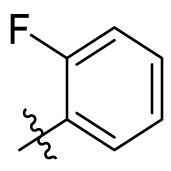 |
0.205 ± 0.033 |
| C9 | 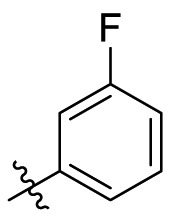 |
0.236 ± 0.018 |
| C10 | 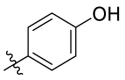 |
0.271 ± 0.018 |
| C11 | 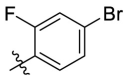 |
0.207 ± 0.024 |
| C12 | 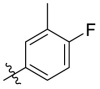 |
0.117 ± 0.016 |
| C13 | 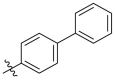 |
1.476 ± 0.117 |
| C14 | 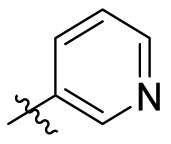 |
0.212 ± 0.024 |
| C15 | 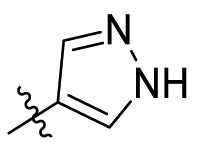 |
0.390 ± 0.003 |
| 19 | 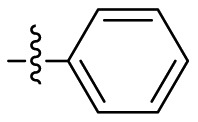 |
1.372 ± 0.047 |
| baicalein (14) | – | 0.966 ± 0.065 |
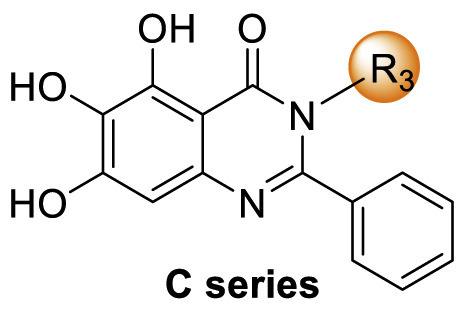
Baicalein was used as the positive control. Inhibitory activity against SARS-CoV-2 Mprowas determined by using the FRET protease activity assay. Values represent a mean ± SD of at least three independent experiments.
