Figure 1: Fragile-like Tregs pervade through blood into the brain giving rise to opioid-induced dysfunction.
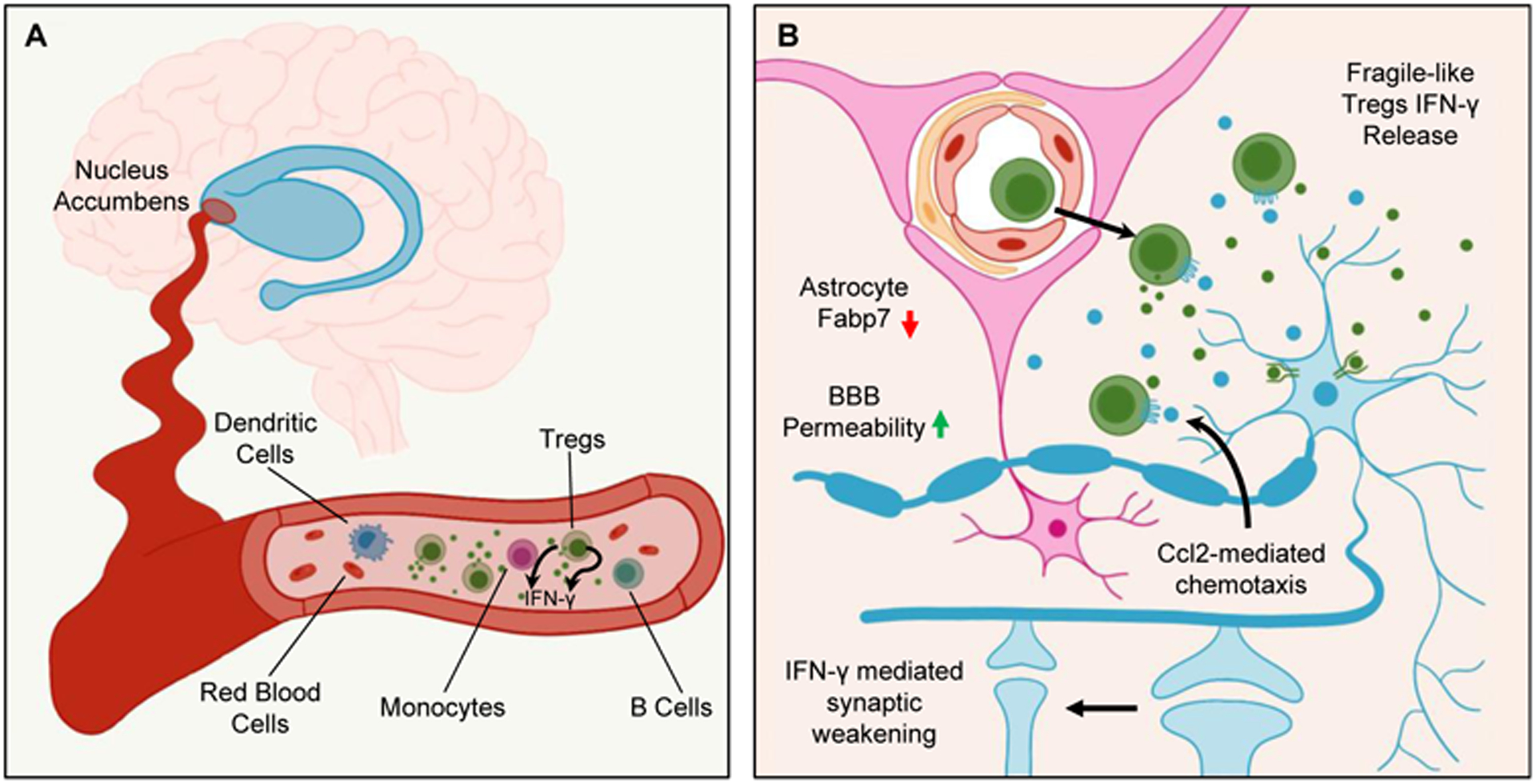
(A) In the blood of both humans and mice, opioids dysregulate the immune system for which this study focuses on Foxp3+ regulatory T cells (Tregs) that falter in their suppression and begin secreting IFN-γ to become “fragile-like.”
(B) Through both chemokine-mediated migration and hyperpermeability of the blood-brain barrier (BBB), these fragile-like Tregs mobilize into the brain, localizing to the nucleus accumbens (NAc). There, they continue to secrete IFN-γ, and by signaling through IFN-γ receptor R1 (Ifngr1) on neurons in the NAc, induce morphological changes that result in synaptic weakening. Blocking IFN-γ signaling prevented synaptic instability and improved withdrawal symptoms. Altogether, these findings emphasize relevant changes occurring at the neuroimmune interface for opioid-induced impairments.
