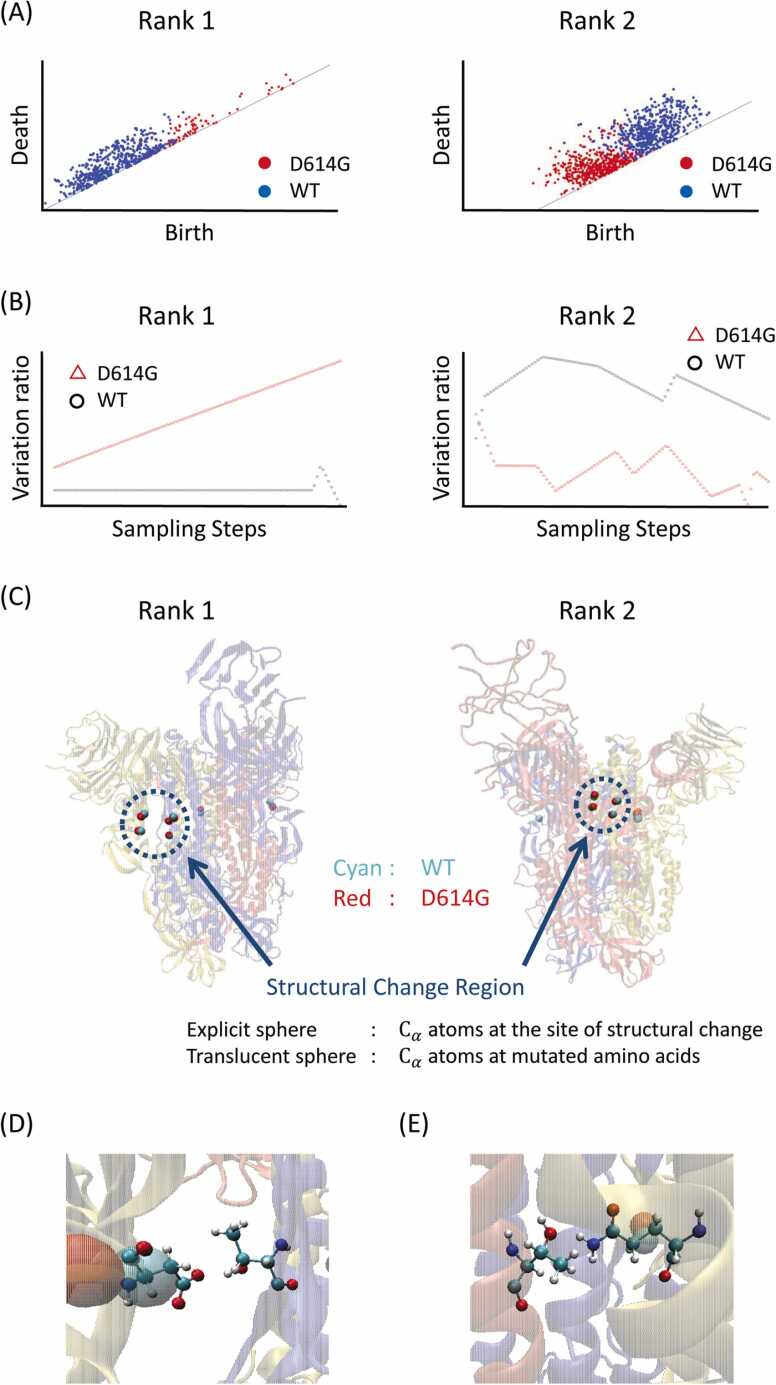
Fig. 2.
Comparison of WT and spiked proteins with the D614G mutation under our analysis framework: (A) The difference in persistent diagram distribution for the most-confirmed change (Rank 1) and second-most confirmed change (Rank 2) between D614G (red) and WT (blue). (B) Smoothed trajectory of the polar distance variation with respect to the accumulation step on the persistent diagram, with D614G and WT indicated by red triangles and black circles, respectively. (C) Structural change sites detected in Rank 1 and Rank 2. The explicit sphere in the dashed blue circle represents the Cα describing the structural change, and the other translucent spheres denote the Cα of the amino acid mutation sites. (D) Location of the most significant changes in the rate of hydrogen bond formation in the thermal vibration sampling of WT and D614G. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)