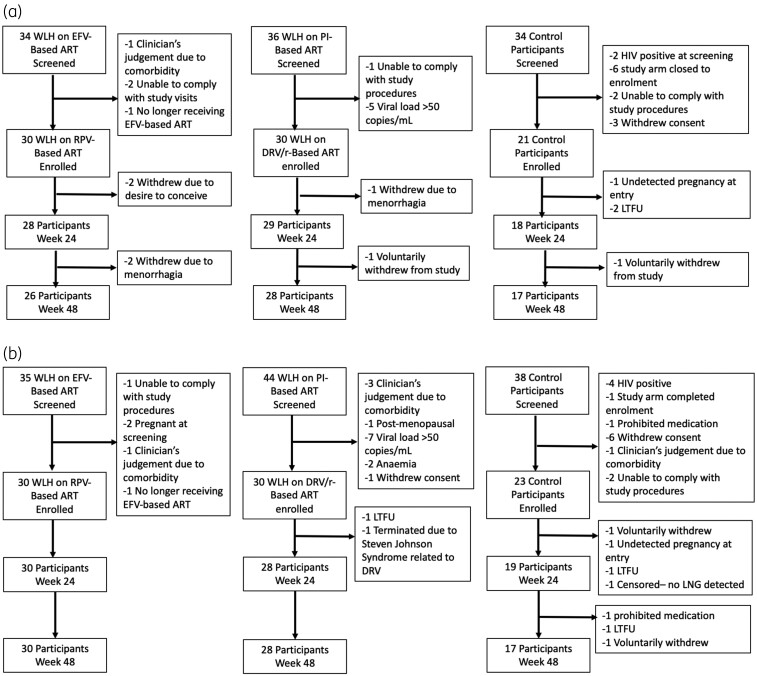
Figure 2.
(a) Participant disposition in the etonogestrel study through the primary endpoint at Week 24 and secondary endpoint at Week 48. EFV, efavirenz; DRV/r, darunavir/ritonavir; RPV, rilpivirine; LTFU, lost to follow-up; WLH, women living with HIV. (b) Participant disposition in the levonorgestrel study through the primary endpoint at Week 24 and secondary endpoint at Week 48. DRV/r, darunavir/ritonavir; EFV, efavirenz; RPV, rilpivirine; LTFU, lost to follow-up; LNG, levonorgestrel; WLH, women living with HIV.