FIGURE 4.
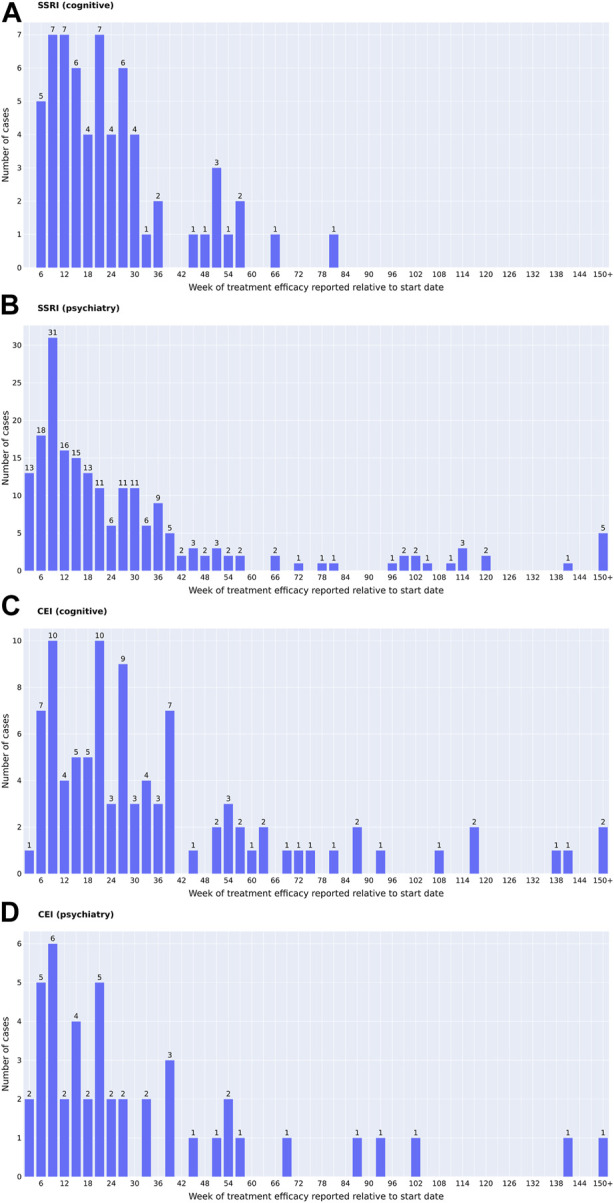
Time of the reported medication start date ((A, B) SSRIs; (C, D) CEIs) to the determination of treatment efficacy ((A, C) change in cognition; (B, D) change in psychiatric symptoms). The time of the reported intolerance cases is not included. One date of reported effect per individual is presented. For patients who had multiple dates of similar treatment effects reported, the date of reported cognitive comments that matched the dates of MMSE changes was prioritized when available. For all the psychiatric effects and the cognitive effects that did not have the matching dates of MMSE changes, the comments that were reported closest to 6 months, or 26 weeks, were prioritized.
